Question: 4. Answer ALL parts. (a) Table below shows the composition of a polluted sample of air which includes the main components of the atmosphere and
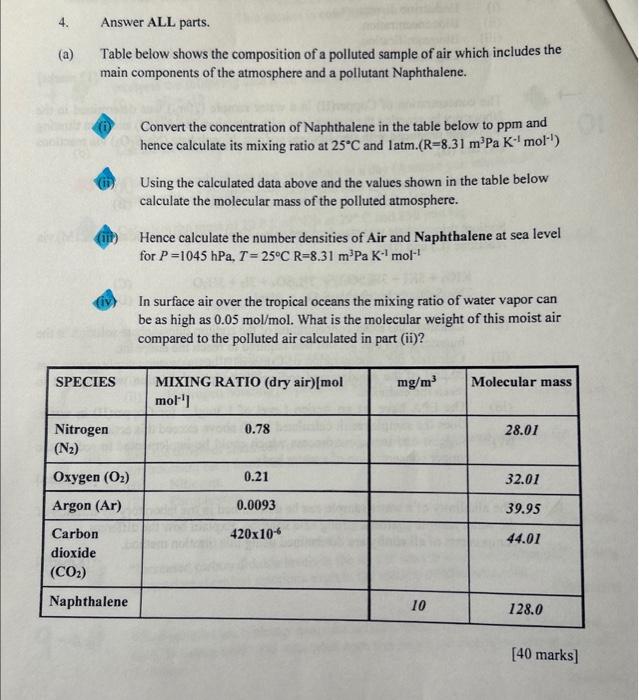
4. Answer ALL parts. (a) Table below shows the composition of a polluted sample of air which includes the main components of the atmosphere and a pollutant Naphthalene. Convert the concentration of Naphthalene in the table below to ppm and hence calculate its mixing ratio at 25C and 1atm.(R=8.31m3PaK1mol1) Using the calculated data above and the values shown in the table below calculate the molecular mass of the polluted atmosphere. Hence calculate the number densities of Air and Naphthalene at sea level for P=1045hPa,T=25CR=8.31m3PaK1mol1 In surface air over the tropical oceans the mixing ratio of water vapor can be as high as 0.05mol/mol. What is the molecular weight of this moist air compared to the polluted air calculated in part (ii)? [40 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


