Question: 4. Answer the following questions in the table below: a. Draw the Lewis structures for the following molecules or ions. (5 marks) b. Identify the
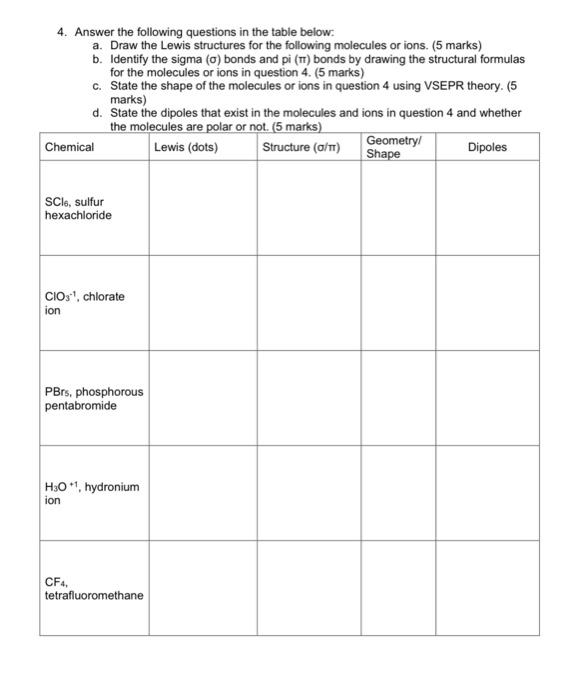
4. Answer the following questions in the table below: a. Draw the Lewis structures for the following molecules or ions. (5 marks) b. Identify the sigma () bonds and pi ( ) bonds by drawing the structural formulas for the molecules or ions in question 4 . ( 5 marks) c. State the shape of the molecules or ions in question 4 using VSEPR theory. (5 marks) d. State the dipoles that exist in the molecules and ions in question 4 and whether
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


