Question: (4) Assuming that the reaction A + B + C + D is irreversible and elementary and goes to completion, determine the reaction order and
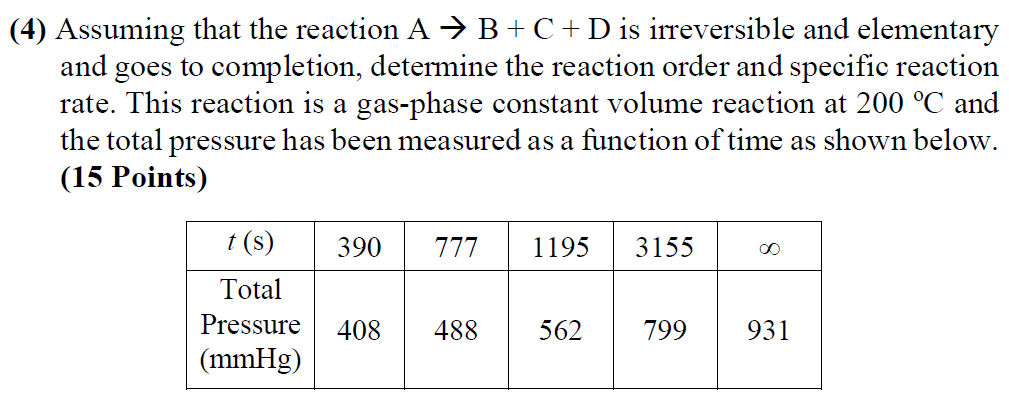
(4) Assuming that the reaction A + B + C + D is irreversible and elementary and goes to completion, determine the reaction order and specific reaction rate. This reaction is a gas-phase constant volume reaction at 200 C and the total pressure has been measured as a function of time as shown below. (15 Points) 390 777 1195 3155 t(s) Total Pressure (mmHg) 408 488 562 799 931
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


