Question: 4. BASIC MANIPULATION & CALCULATION (EASY). The Nernst potential is often employed by experimentalists as a quick-and-dirty way to estimate a particular environment. At the
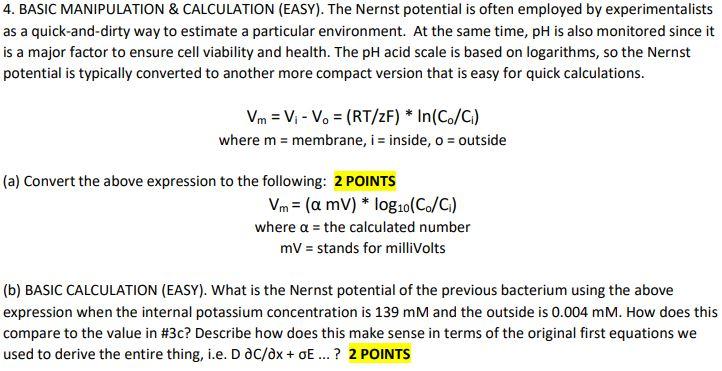
4. BASIC MANIPULATION & CALCULATION (EASY). The Nernst potential is often employed by experimentalists as a quick-and-dirty way to estimate a particular environment. At the same time, pH is also monitored since it is a major factor to ensure cell viability and health. The pH acid scale is based on logarithms, so the Nernst potential is typically converted to another more compact version that is easy for quick calculations. Vm = V - V. = (RT/ZF) * In(Co/C) where m = membrane, i = inside, o = outside (a) Convert the above expression to the following: 2 POINTS Vm= (a mv) * log10 (C/C) where a = the calculated number mV = stands for millivolts (b) BASIC CALCULATION (EASY). What is the Nernst potential of the previous bacterium using the above expression when the internal potassium concentration is 139 mM and the outside is 0.004 mM. How does this compare to the value in #3c? Describe how does this make sense in terms of the original first equations we used to derive the entire thing, i.e. D DC/ax+oE ...? 2 POINTS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


