Question: 4. Competing technology A competing technology is based on the gas phase reversible reaction E 2 S+H The reaction rate is given (in terms

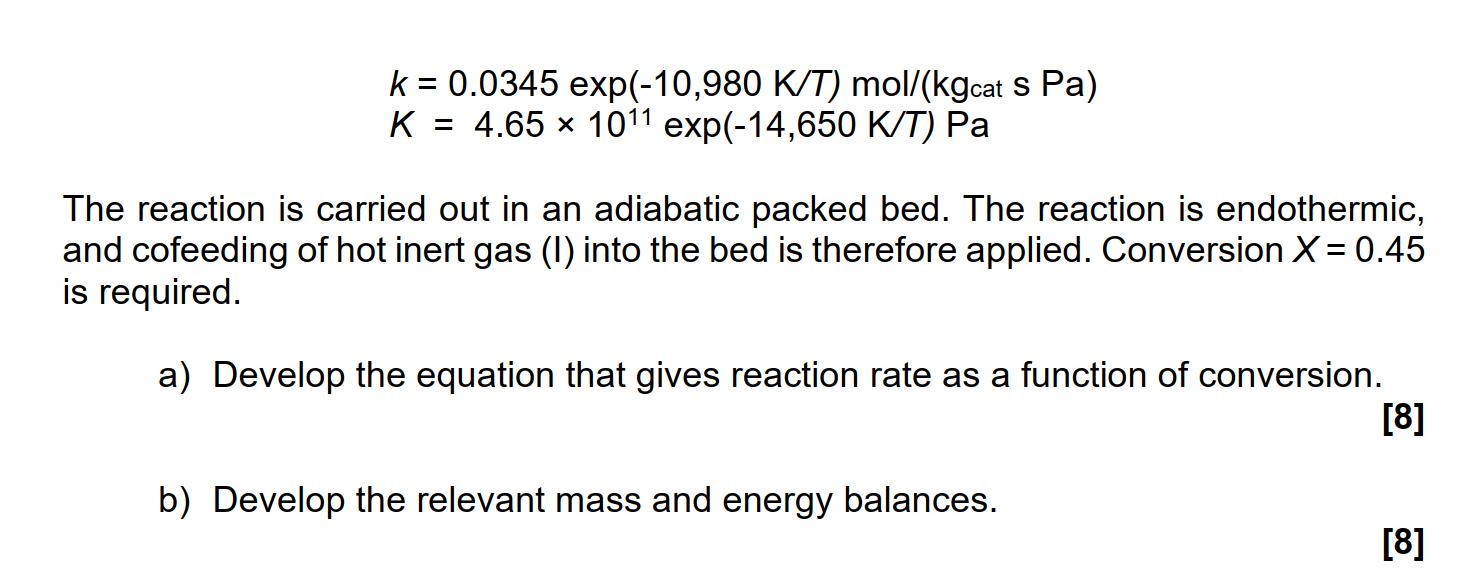
4. Competing technology A competing technology is based on the gas phase reversible reaction E 2 S+H The reaction rate is given (in terms of partial pressures) by r = = k(P - Ps P/ K) in which the rate constant k and the equilibrium constant K are given by k = 0.0345 exp(-10,980 K/T) mol/(kgcat s Pa) K = 4.65 1011 exp(-14,650 K/T) Pa The reaction is carried out in an adiabatic packed bed. The reaction is endothermic, and cofeeding of hot inert gas (I) into the bed is therefore applied. Conversion X = 0.45 is required. a) Develop the equation that gives reaction rate as a function of conversion. b) Develop the relevant mass and energy balances. [8] [8]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


