Question: 4. Consider a dilute solution with a total number Ntot of solute molecules contained in a total volume Vtot. The total number density of solutes
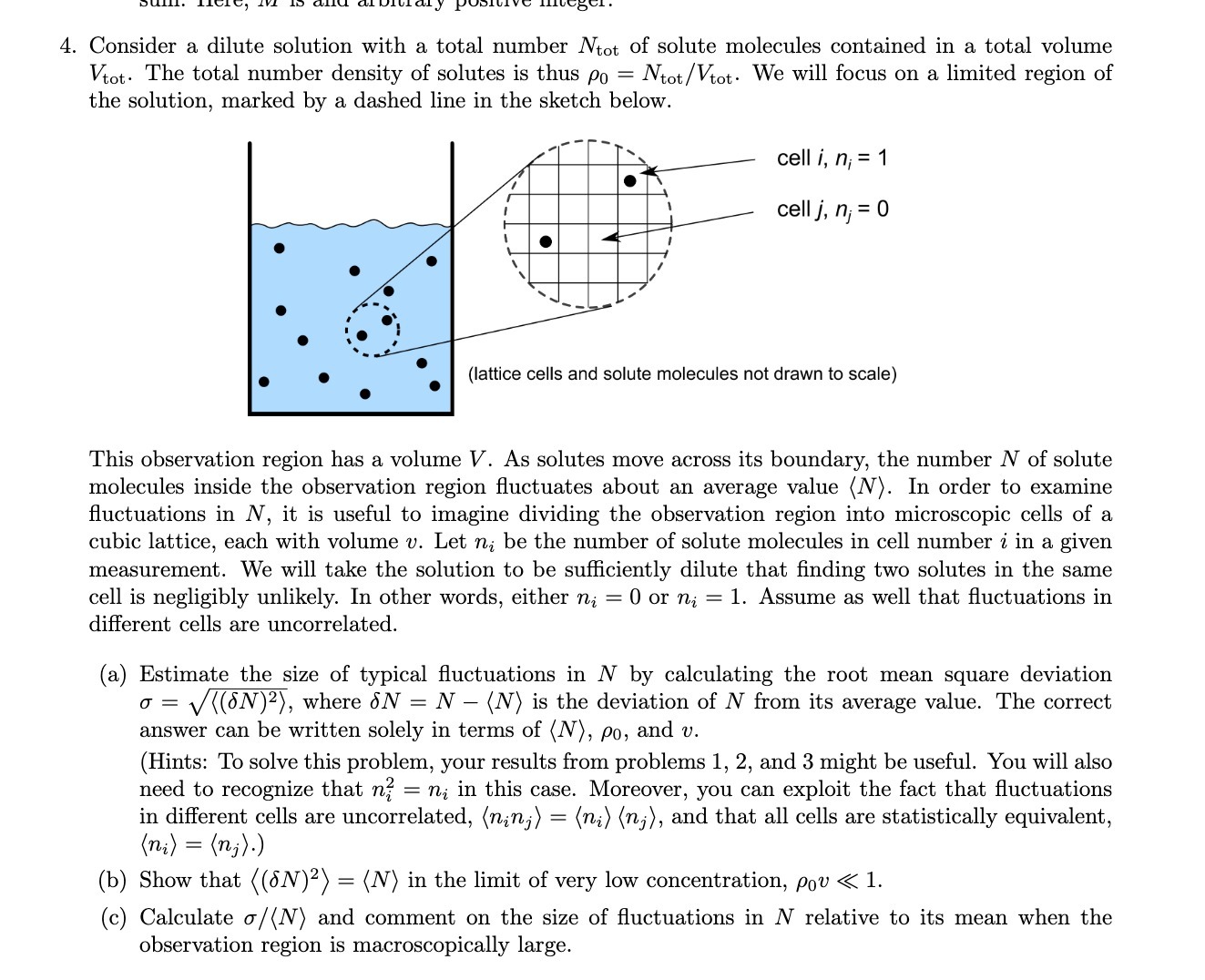
4. Consider a dilute solution with a total number Ntot of solute molecules contained in a total volume Vtot. The total number density of solutes is thus po = Ntot/Vtot. We will focus on a limited region of the solution, marked by a dashed line in the sketch below. cell i, n; = 1 cell j, n; = 0 (lattice cells and solute molecules not drawn to scale) This observation region has a volume V. As solutes move across its boundary, the number N of solute molecules inside the observation region fluctuates about an average value (N). In order to examine fluctuations in N, it is useful to imagine dividing the observation region into microscopic cells of a cubic lattice, each with volume v. Let ni be the number of solute molecules in cell number i in a given measurement. We will take the solution to be sufficiently dilute that finding two solutes in the same cell is negligibly unlikely. In other words, either ni = 0 or ni = 1. Assume as well that fluctuations in different cells are uncorrelated. (a) Estimate the size of typical fluctuations in N by calculating the root mean square deviation o = ((ON)2), where ON = N - (N) is the deviation of N from its average value. The correct answer can be written solely in terms of (N), po, and v. (Hints: To solve this problem, your results from problems 1, 2, and 3 might be useful. You will also need to recognize that n? = ni in this case. Moreover, you can exploit the fact that fluctuations in different cells are uncorrelated, (nin;) = (ni) (n;), and that all cells are statistically equivalent, ( ni) = (n; ). ) (b) Show that ( (ON)2) = (N) in the limit of very low concentration, Pou
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


