Question: 4. Consider the data presented in Exercise 2 above. (a) By using appropriate graphs, determine whether the reaction is first order or second order. (b)

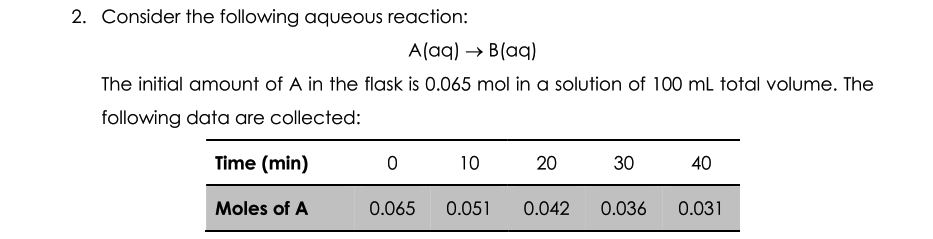
4. Consider the data presented in Exercise 2 above. (a) By using appropriate graphs, determine whether the reaction is first order or second order. (b) Calculate the value of the rate constant for the reaction. (c) Calculate the half-life for the reaction. 2. Consider the following aqueous reaction: Alaq) B(aq) The initial amount of A in the flask is 0.065 mol in a solution of 100 ml total volume. The following data are collected: Time (min) 0 10 20 30 40 Moles of A 0.065 0.051 0.042 0.036 0.031
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


