Question: 4. Consider the following PES for an unknown element X 2 151 Relative # 100 electrons 500 1 2 6 2 12.1 0.58 1.09 7.19
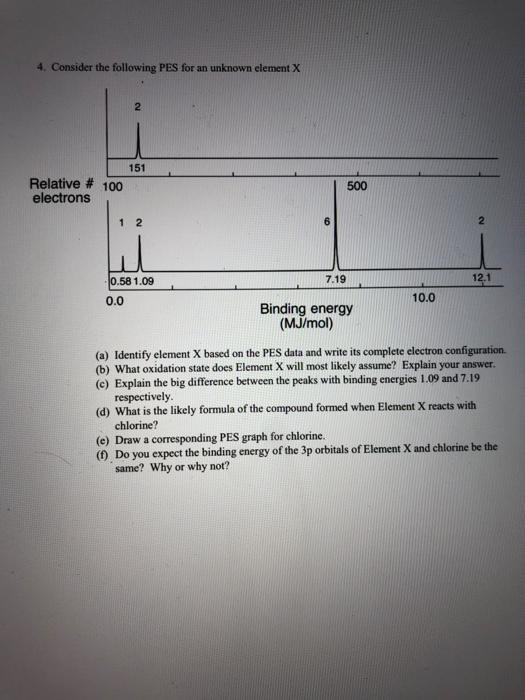
4. Consider the following PES for an unknown element X 2 151 Relative # 100 electrons 500 1 2 6 2 12.1 0.58 1.09 7.19 0.0 10.0 Binding energy (MJ/mol) (a) Identify element X based on the PES data and write its complete electron configuration (b) What oxidation state does Element X will most likely assume? Explain your answer. (e) Explain the big difference between the peaks with binding energies 1.09 and 7.19 respectively (d) What is the likely formula of the compound formed when Element X reacts with chlorine? (e) Draw a corresponding PES graph for chlorine. (1) Do you expect the binding energy of the 3p orbitals of Element X and chlorine be the same? Why or why not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


