Question: 4. Explain the rationale why excess cyanide is desired during gold cyanide leaching and how excess cyanide can affect gold recovery from leaching solution. (10
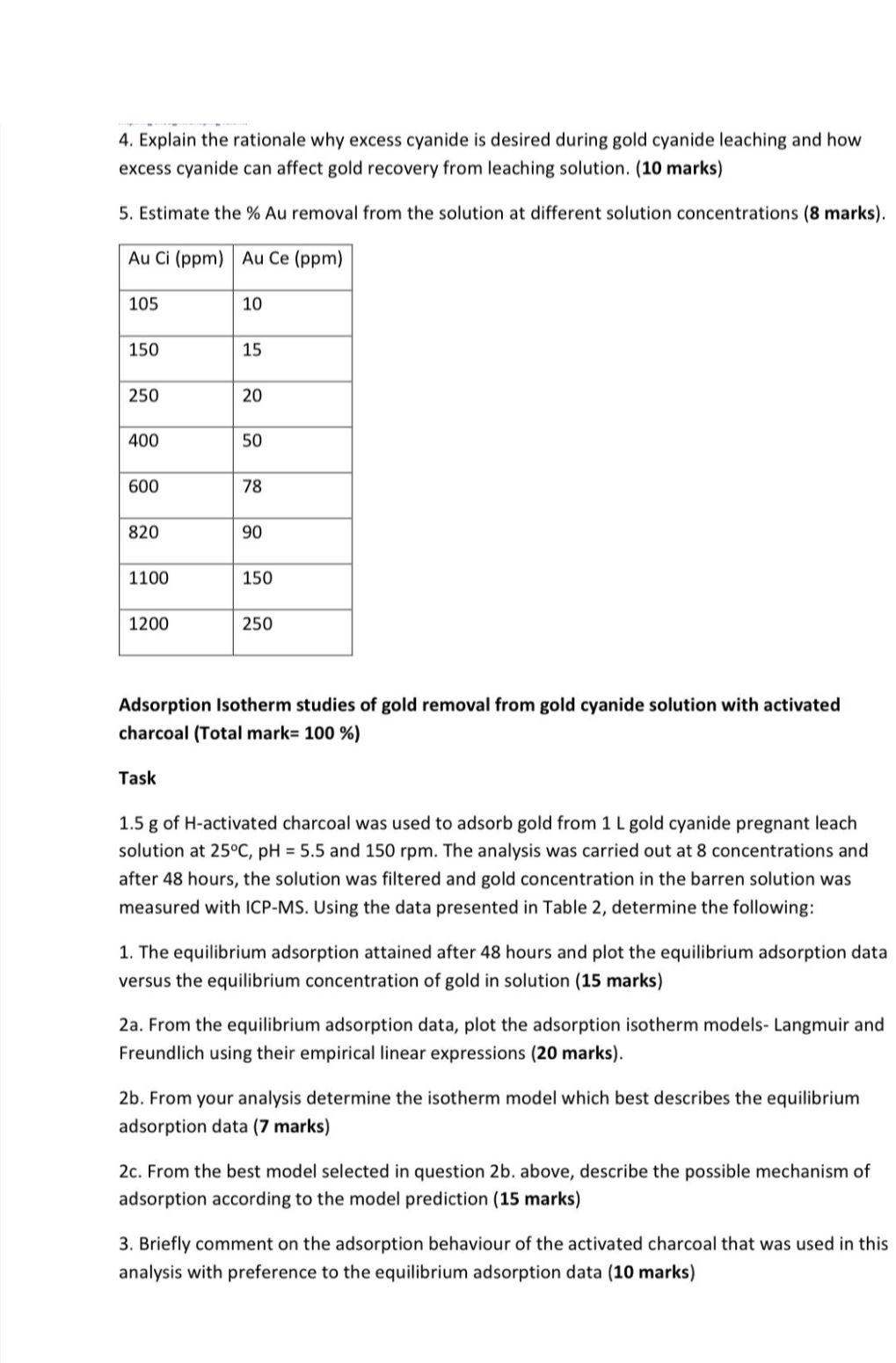
4. Explain the rationale why excess cyanide is desired during gold cyanide leaching and how excess cyanide can affect gold recovery from leaching solution. (10 marks) 5. Estimate the \% Au removal from the solution at different solution concentrations (8 marks). Adsorption Isotherm studies of gold removal from gold cyanide solution with activated charcoal (Total mark 100%) Task 1.5g of H-activated charcoal was used to adsorb gold from 1L gold cyanide pregnant leach solution at 25C,pH=5.5 and 150rpm. The analysis was carried out at 8 concentrations and after 48 hours, the solution was filtered and gold concentration in the barren solution was measured with ICP-MS. Using the data presented in Table 2, determine the following: 1. The equilibrium adsorption attained after 48 hours and plot the equilibrium adsorption data versus the equilibrium concentration of gold in solution (15 marks) 2a. From the equilibrium adsorption data, plot the adsorption isotherm models- Langmuir and Freundlich using their empirical linear expressions (20 marks). 2b. From your analysis determine the isotherm model which best describes the equilibrium adsorption data (7 marks) 2c. From the best model selected in question 2b. above, describe the possible mechanism of adsorption according to the model prediction (15 marks) 3. Briefly comment on the adsorption behaviour of the activated charcoal that was used in this analysis with preference to the equilibrium adsorption data (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


