Question: 4. Here's a question from a previous final exam, which many people missed. The tungsten dimer molecule, W2 , has the molecular orbital diagram shown
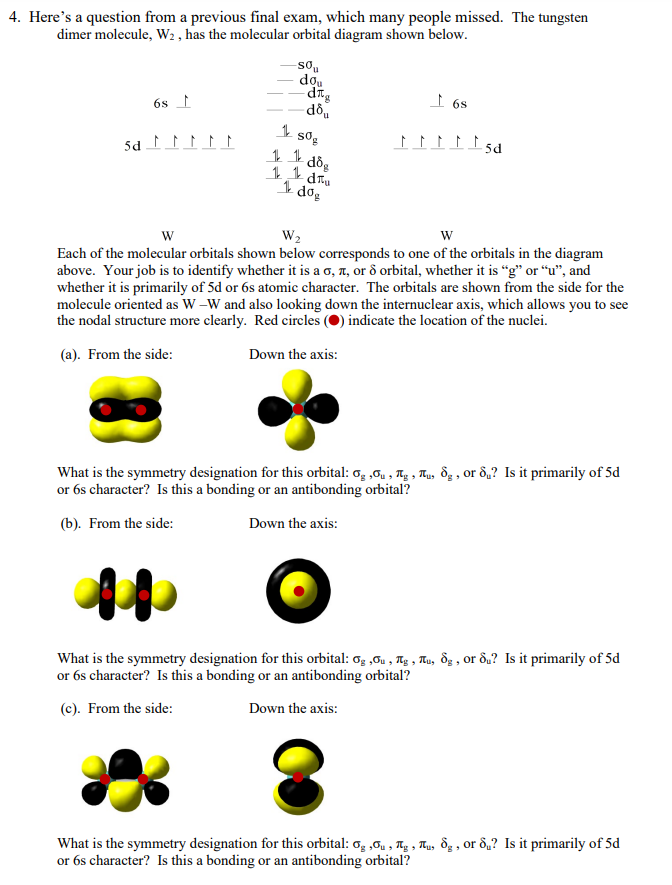
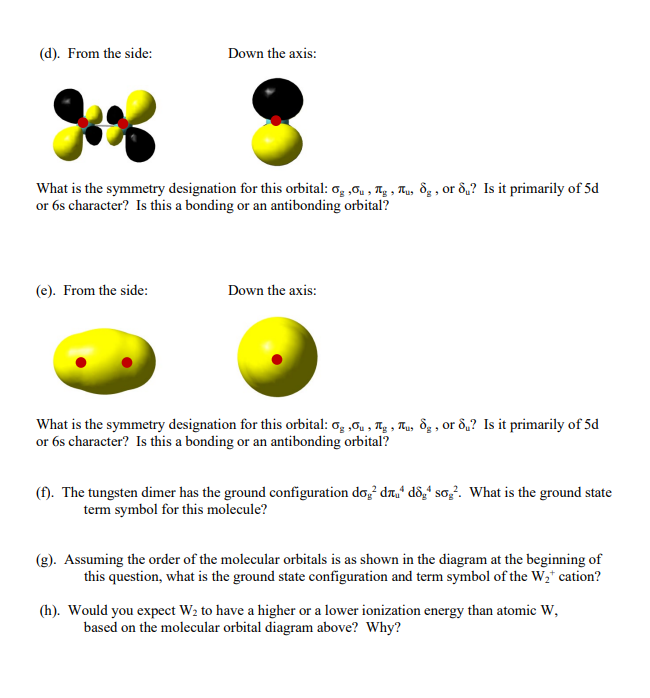
4. Here's a question from a previous final exam, which many people missed. The tungsten dimer molecule, W2 , has the molecular orbital diagram shown below. 6s I 6s so doa -d d8 1 Sog 11 5d Irril lllsa dog The 1 dru 1 dog w W2 W Each of the molecular orbitals shown below corresponds to one of the orbitals in the diagram above. Your job is to identify whether it is a g, n, or 8 orbital, whether it is g or u, and whether it is primarily of 5d or 6s atomic character. The orbitals are shown from the side for the molecule oriented as W-W and also looking down the internuclear axis, which allows you to see the nodal structure more clearly. Red circles() indicate the location of the nuclei. (a). From the side: Down the axis: What is the symmetry designation for this orbital: 0, Qu, Tg, Tu, og, or 8,? Is it primarily of 5d or 6s character? Is this a bonding or an antibonding orbital? (b). From the side: Down the axis: What is the symmetry designation for this orbital: 0g, Ou, Tg, Ttu, og, or 8,? Is it primarily of 5d or 6s character? Is this a bonding or an antibonding orbital? (C). From the side: Down the axis: 9 What is the symmetry designation for this orbital: 04, Ou , Tg, Tu, og, or 8,? Is it primarily of 5d or 6s character? Is this a bonding or an antibonding orbital? (d). From the side: Down the axis: What is the symmetry designation for this orbital: 0,,Ou s Tig, Tu, 8g, or 8,? Is it primarily of 5d or 6s character? Is this a bonding or an antibonding orbital? (e). From the side: Down the axis: What is the symmetry designation for this orbital: 0,0u, Tg, Tu, og, or 8,? Is it primarily of 5d or 6s character? Is this a bonding or an antibonding orbital? (f). The tungsten dimer has the ground configuration do 2 dny* d8,4 sog. What is the ground state term symbol for this molecule? (g). Assuming the order of the molecular orbitals is as shown in the diagram at the beginning of this question, what is the ground state configuration and term symbol of the Wat cation? (h). Would you expect W2 to have a higher or a lower ionization energy than atomic W, based on the molecular orbital diagram above? Why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


