Question: 4. Human blood is slightly basic in nature with a normal pH range of 7.35-7.45. The blood pH is maintained by the carbonic acid-bicarbonate
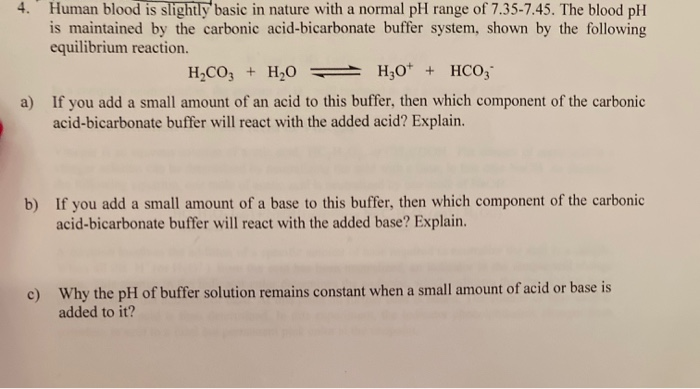
4. Human blood is slightly basic in nature with a normal pH range of 7.35-7.45. The blood pH is maintained by the carbonic acid-bicarbonate buffer system, shown by the following equilibrium reaction. HCO3 + HO H3O+ + HCO3 a) If you add a small amount of an acid to this buffer, then which component of the carbonic acid-bicarbonate buffer will react with the added acid? Explain. b) If you add a small amount of a base to this buffer, then which component of the carbonic acid-bicarbonate buffer will react with the added base? Explain. c) Why the pH of buffer solution remains constant when a small amount of acid or base is added to it?
Step by Step Solution
3.33 Rating (150 Votes )
There are 3 Steps involved in it
Answer a The component of the buffer that will react to the added acid has to be determined and expl... View full answer

Get step-by-step solutions from verified subject matter experts


