Question: 4. In class, we learnt that sulfur dioxide (SO2) is an air pollutant that is commonly monitored by environmental agencies worldwide. SO2 contributes to acid
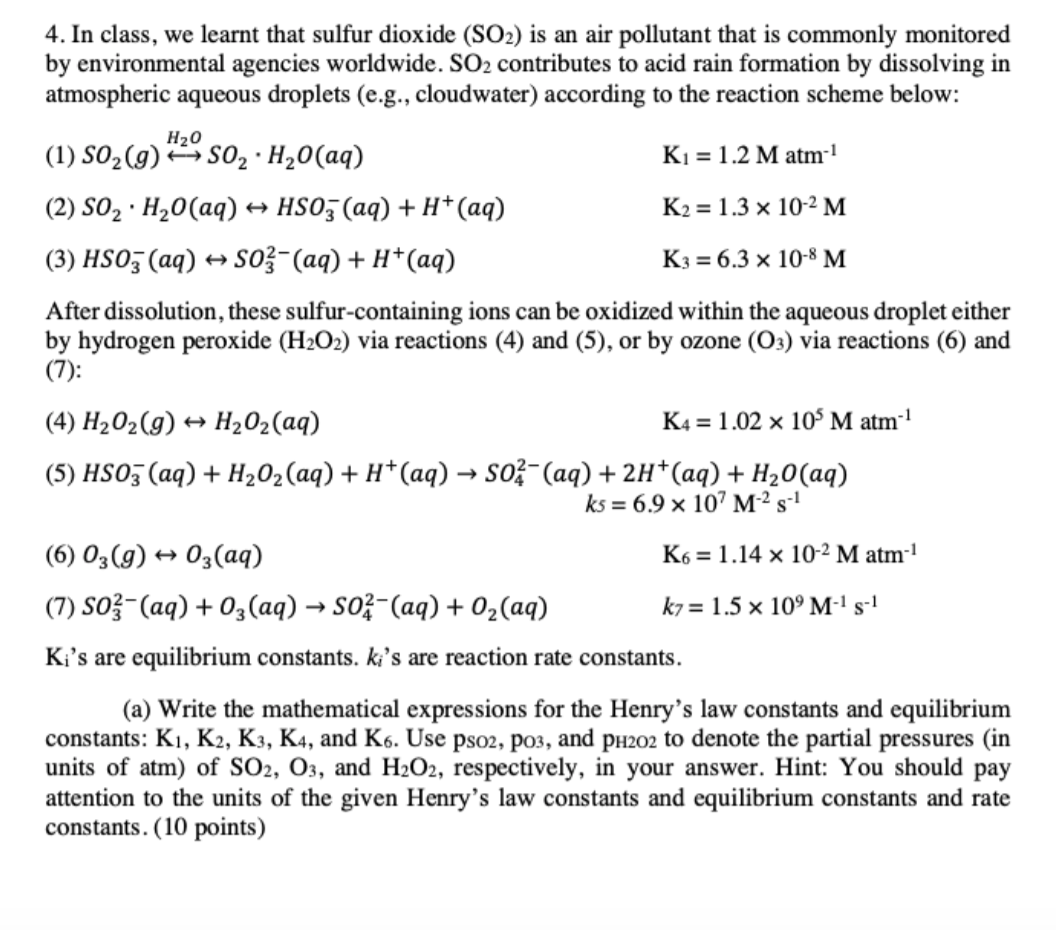
4. In class, we learnt that sulfur dioxide (SO2) is an air pollutant that is commonly monitored by environmental agencies worldwide. SO2 contributes to acid rain formation by dissolving in atmospheric aqueous droplets (e.g., cloudwater) according to the reaction scheme below: H2O > (1) SO2(g) 2302H20(aq) K1 = 1.2 M atm-1 (2) SO2: H20(aq) + HSO3(aq) + H+ (aq) K2 = 1.3 x 10-2 M (3) HS03 (aq) + S03-(aq) + H+(aq) K3 = 6.3 x 10-8 M After dissolution, these sulfur-containing ions can be oxidized within the aqueous droplet either by hydrogen peroxide (H2O2) via reactions (4) and (5), or by ozone (03) via reactions (6) and (7): (4) H202(9) + H202(aq) K4 = 1.02 x 10M atm (5) HS03 (aq) + H2O2(aq) + H+(aq) S02-(aq) + 2H+(aq) + H2O(aq) ks = 6.9 x 107 M25-1 (6) 03(9) + 03(aq) K6 = 1.14 x 10-2 M atm-1 (7) So}-(aq) + 03(aq) 04-(aq) + O2(aq) kz= 1.5 x 10'M-15-1 Ki's are equilibrium constants. ki's are reaction rate constants. (a) Write the mathematical expressions for the Henry's law constants and equilibrium constants: K1, K2, K3, K4, and K6. Use pso2, po3, and PH202 to denote the partial pressures in units of atm) of SO2, O3, and H2O2, respectively, in your answer. Hint: You should pay attention to the units of the given Henry's law constants and equilibrium constants and rate constants. (10 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


