Question: 4. Nylon 6, 6 has been prepared through step-growth polymerization (Water is the byproduct not in the unbalanced reaction scheme): shown up ki H O=D
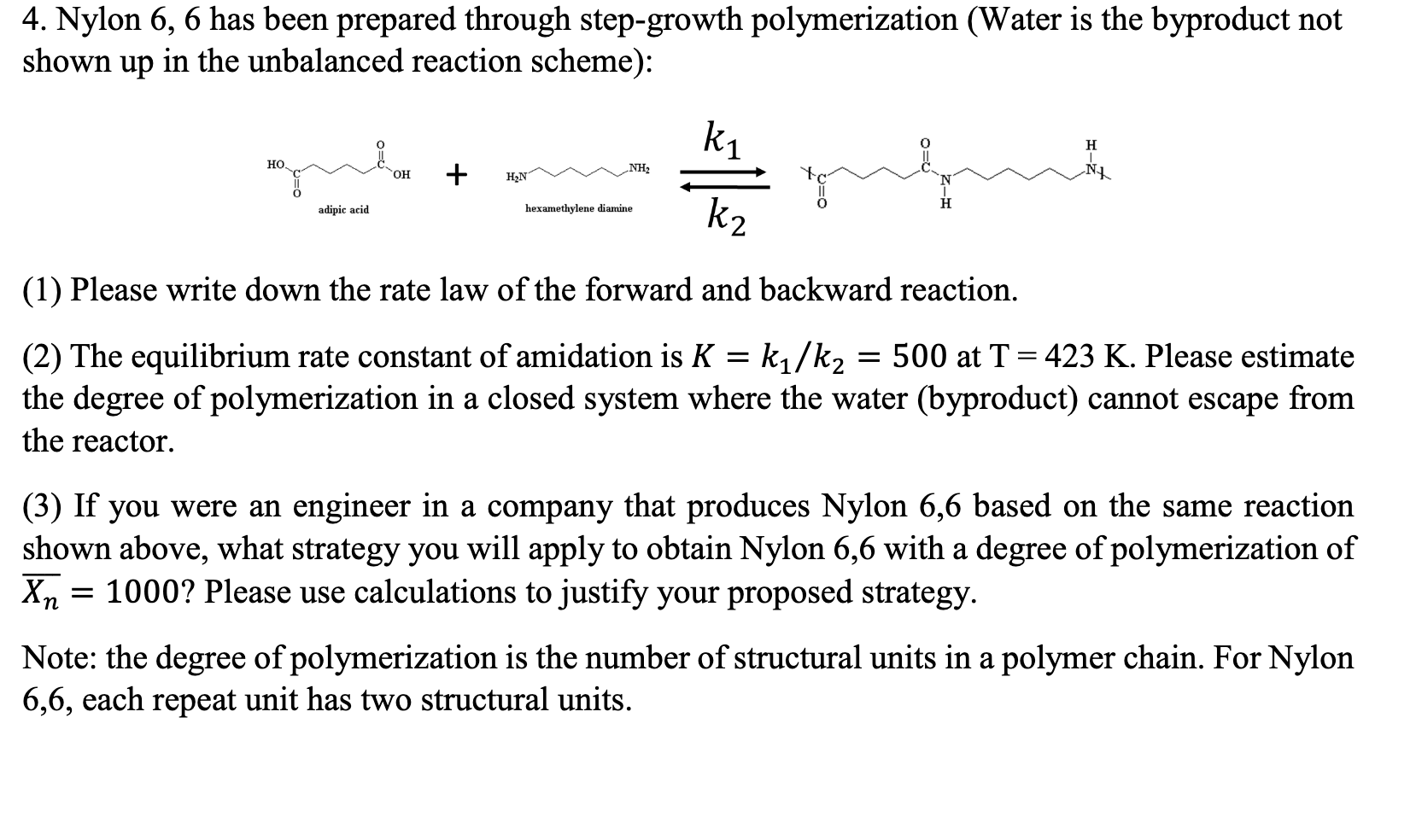
4. Nylon 6, 6 has been prepared through step-growth polymerization (Water is the byproduct not in the unbalanced reaction scheme): shown up ki H O=D , OH + NH2 HON NA adipic acid hexamethylene diamine k2 (1) Please write down the rate law of the forward and backward reaction. = (2) The equilibrium rate constant of amidation is K = k/k2 = 500 at T = 423 K. Please estimate the degree of polymerization in a closed system where the water (byproduct) cannot escape from the reactor. (3) If you were an engineer in a company that produces Nylon 6,6 based on the same reaction shown above, what strategy you will apply to obtain Nylon 6,6 with a degree of polymerization of X = 1000? Please use calculations to justify your proposed strategy. = Note: the degree of polymerization is the number of structural units in a polymer chain. For Nylon 6,6, each repeat unit has two structural units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


