Question: 4. The liquid-phase reaction A+2BC+D is conducted in a CSTR. The reaction kinetics are first-order in both A and B with a rate constant kA=0.17L/molmin.
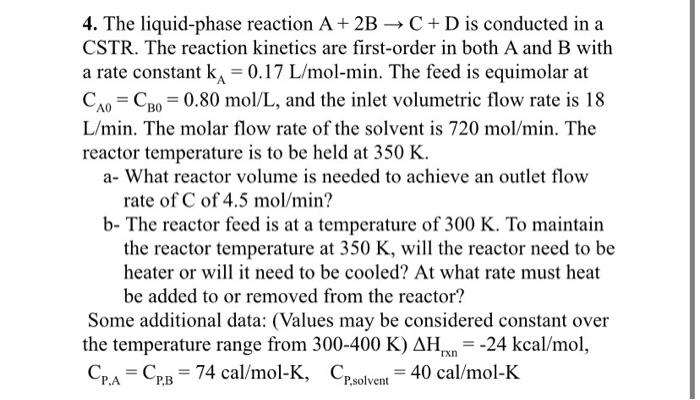
4. The liquid-phase reaction A+2BC+D is conducted in a CSTR. The reaction kinetics are first-order in both A and B with a rate constant kA=0.17L/molmin. The feed is equimolar at CA0=CB0=0.80mol/L, and the inlet volumetric flow rate is 18 L/min. The molar flow rate of the solvent is 720mol/min. The reactor temperature is to be held at 350K. a- What reactor volume is needed to achieve an outlet flow rate of C of 4.5mol/min ? b- The reactor feed is at a temperature of 300K. To maintain the reactor temperature at 350K, will the reactor need to be heater or will it need to be cooled? At what rate must heat be added to or removed from the reactor? Some additional data: (Values may be considered constant over the temperature range from 300400K)Hrxn=24kcal/mol, CP,A=CP,B=74cal/molK,CP,solvent=40cal/molK
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


