Question: 4. The monomer unit for poly(acrylic acid) (PAA) is given in the diagram below. In water, the PAA acid group (COOH) is fully protonated at
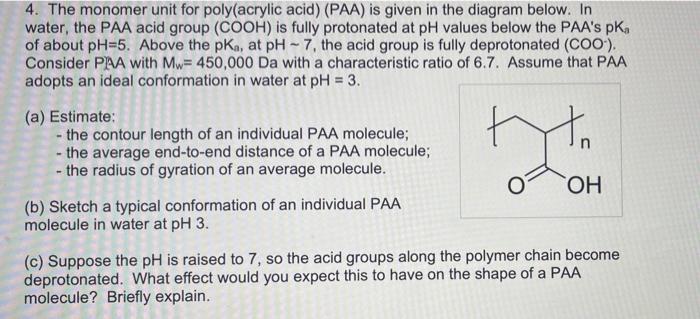
4. The monomer unit for poly(acrylic acid) (PAA) is given in the diagram below. In water, the PAA acid group (COOH) is fully protonated at pH values below the PAA's PK of about pH=5. Above the pka, at pH - 7, the acid group is fully deprotonated (COO), Consider PAA with Mw= 450,000 Da with a characteristic ratio of 6.7. Assume that PAA adopts an ideal conformation in water at pH = 3. yo (a) Estimate: - the contour length of an individual PAA molecule; - the average end-to-end distance of a PAA molecule; - the radius of gyration of an average molecule. (b) Sketch a typical conformation of an individual PAA molecule in water at pH 3. OH (c) Suppose the pH is raised to 7, so the acid groups along the polymer chain become deprotonated. What effect would you expect this to have on the shape of a PAA molecule? Briefly explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


