Question: 4. The standard electrode potentialfor Aluminum is found in Appendix 13 of the e-book (page 1107). Find the potentialif the concentration of the ionsin the
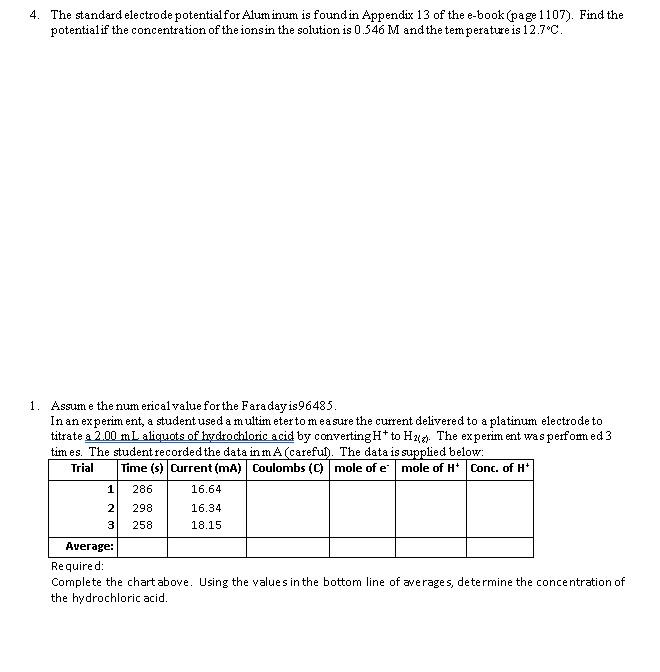
4. The standard electrode potentialfor Aluminum is found in Appendix 13 of the e-book (page 1107). Find the potentialif the concentration of the ionsin the solution is 0.546 M and the temperature is 12.7C. 1 1. Assume the numerical value for the Faraday is96485. In an experiment, a student used a multimeter to measure the current delivered to a platinum electrode to titrate a 2.00 mL aliquots of hydrochloric acid by converting H+ to H20. The experiment was pert ed3 times. The student recorded the data in mA (careful). The data is supplied below: Trial Time (s) Current (mA) Coulombs (C) mole of e mole of H* Conc. of Hi 286 16.64 298 16.34 258 18.15 Average: Required: Complete the chart above. Using the values in the bottom line of averages, determine the concentration of the hydrochloric acid 2 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


