Question: 4. What is the difference between diluted and concentrated buffers? 5. Which solution shows more capacity? 6. What is the extent of closeness of the

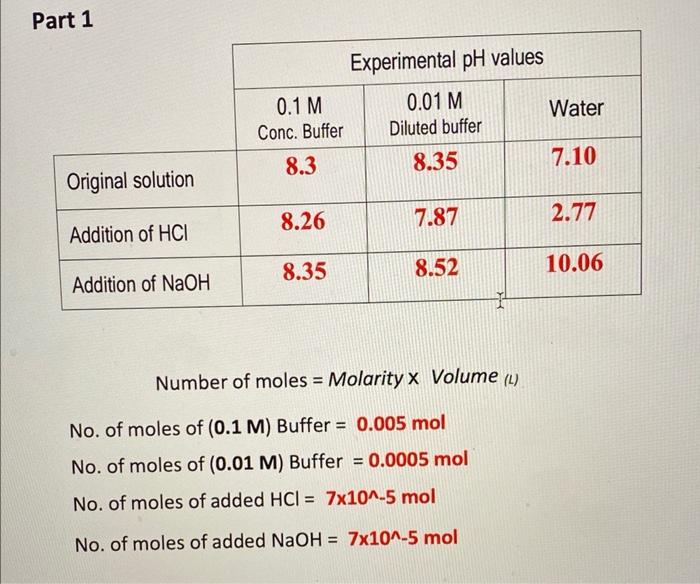
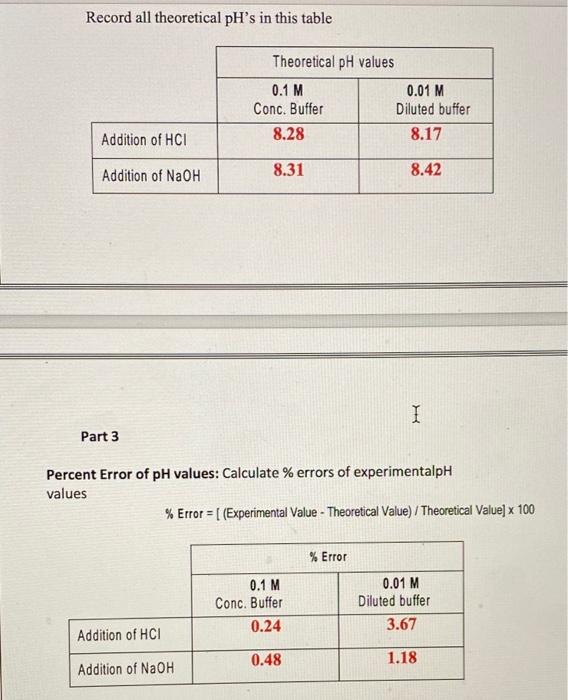
4. What is the difference between diluted and concentrated buffers? 5. Which solution shows more capacity? 6. What is the extent of closeness of the measured pH value and the theoretical values? 7. Give a justification for high \% Error if any? Number of moles = Molarity Volume (L) No. of moles of (0.1M) Buffer =0.005mol No. of moles of (0.01 M) Buffer =0.0005mol No. of moles of added HCl=7105mol No. of moles of added NaOH=7105 mol Record all theoretical pH's in this table Part 3 Percent Error of pH values: Calculate \% errors of experimentalpH values %Error=[(ExperimentalValue-TheoreticalValue)/TheoreticalValue]100
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


