Question: 4) You want to prepare a buffer solution with a pH of 4.500. Which of the following weak acids/would be suitable to use? WhichofthefollowingweakHIO3Ka=1.6101HCOKa=1.4103CH3COOHKa=1.8105HCOOHKa=1.8104HClOKa=3.0108HFKa=6.6104
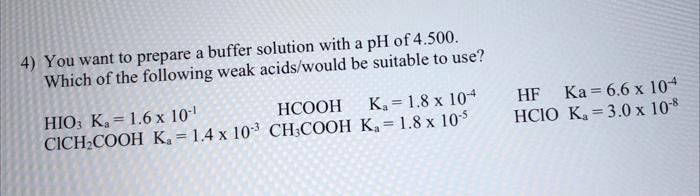
4) You want to prepare a buffer solution with a pH of 4.500. Which of the following weak acids/would be suitable to use? WhichofthefollowingweakHIO3Ka=1.6101HCOKa=1.4103CH3COOHKa=1.8105HCOOHKa=1.8104HClOKa=3.0108HFKa=6.6104
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


