Question: 4.(15 points) Answer the following questions based on the temperature-composition phase diagram of the cyclohexane-toluene system on the right. b) A solution contains 20% of
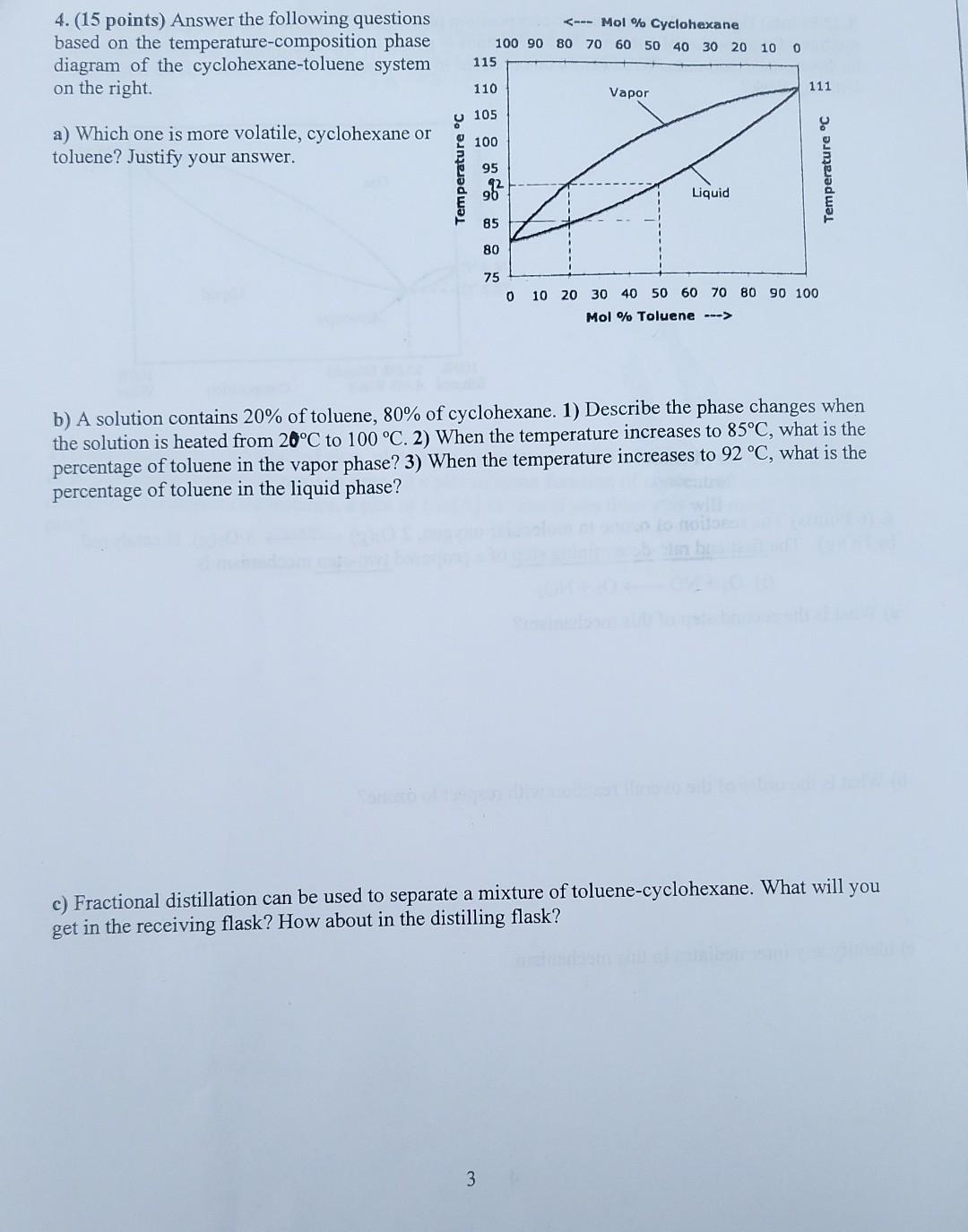
4.(15 points) Answer the following questions based on the temperature-composition phase diagram of the cyclohexane-toluene system on the right. b) A solution contains 20% of toluene, 80% of cyclohexane. 1) Describe the phase changes when the solution is heated from 20C to 100 C. 2) When the temperature increases to 85C, what is the percentage of toluene in the vapor phase? 3) When the temperature increases to 92 C, what is the percentage of toluene in the liquid phase? c) Fractional distillation can be used to separate a mixture of toluene-cyclohexane. What will you get in the receiving flask? How about in the distilling flask? 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


