Question: 42) Calculate the normality, for acid-base work, of a solution prepared by dissolving 6.30 g of barium hydroxide octahydrate [Ba(OH)2.8H20, M = 315.5] in
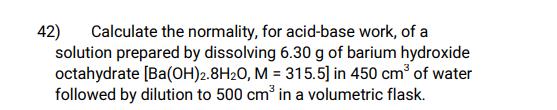
42) Calculate the normality, for acid-base work, of a solution prepared by dissolving 6.30 g of barium hydroxide octahydrate [Ba(OH)2.8H20, M = 315.5] in 450 cm of water followed by dilution to 500 cm in a volumetric flask.
Step by Step Solution
3.32 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


