Question: 4.3. Al 2 O 3 will form a limited solid solution in MgO. At the eutectic temperature (1995C), approximately 18wt% of AI2O3 is soluble in
4.3. Al2O3 will form a limited solid solution in MgO. At the eutectic temperature (1995C), approximately 18wt% of AI2O3 is soluble in MgO. The unit-cell dimensions of MgO decrease. Predict the change in density on the basis of (a) interstitial Al3+ ions and (b) substitutional Al3+ ions.
4.6. Estimate the number of free vacancies, interstitials, and associates in 1cm3 at 500C of (a) pure AgBr and (b) AgBr+ 10-4m/o CdBr.
4.7. Construct a diagram similar to Fig.4.8 for the AgBr data in Problem 4.6.
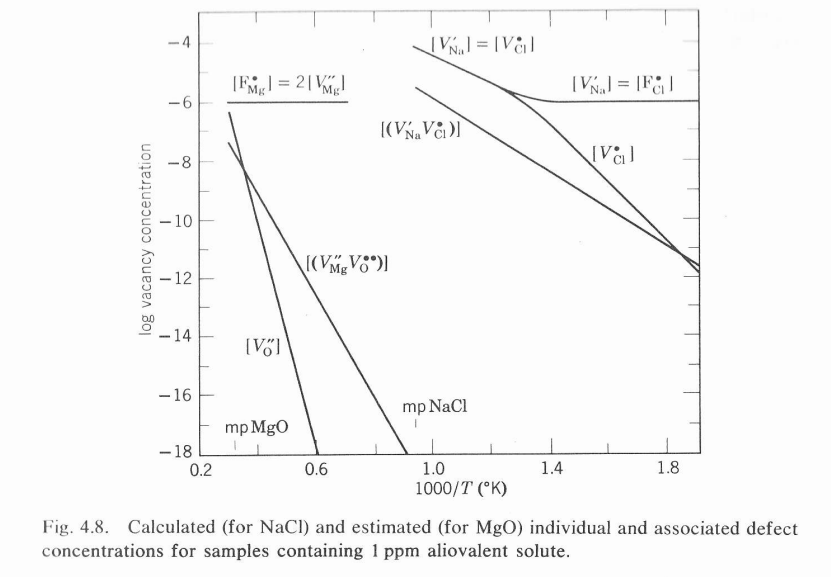
Fig. 4.8. Calculated (for NaCl ) and estimated (for MgO ) individual and associated defect concentrations for samples containing 1ppm aliovalent solute
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


