Question: 4.5 A set of normalized and mutually orthogonal p-state wavefunctions for an atom can be written in the form: P.-xf() ,- yf(). Consider the linear
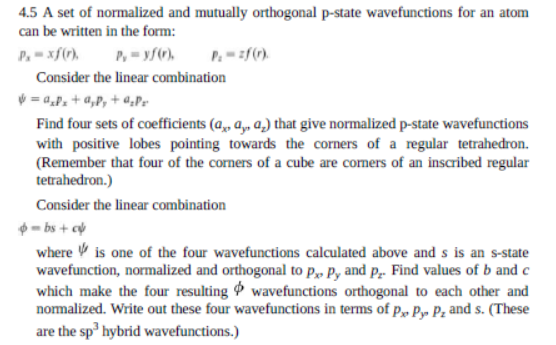
4.5 A set of normalized and mutually orthogonal p-state wavefunctions for an atom can be written in the form: P.-xf() ,- yf(). Consider the linear combination = 0.P. +4,P, +4.P. Find four sets of coefficients (Qx, , 2,) that give normalized p-state wavefunctions with positive lobes pointing towards the corners of a regular tetrahedron. (Remember that four of the corners of a cube are comers of an inscribed regular tetrahedron.) Consider the linear combination - bs + where is one of the four wavefunctions calculated above and s is an s-state wavefunction, normalized and orthogonal to Px P, and Pe. Find values of b and c which make the four resulting wavefunctions orthogonal to each other and normalized. Write out these four wavefunctions in terms of Pe Py: P, and s. (These are the sphybrid wavefunctions.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


