Question: 4.9. The following data were obtained for a rainfall sample collected at a site on your campus: The measured p2H of the solution was 4.05
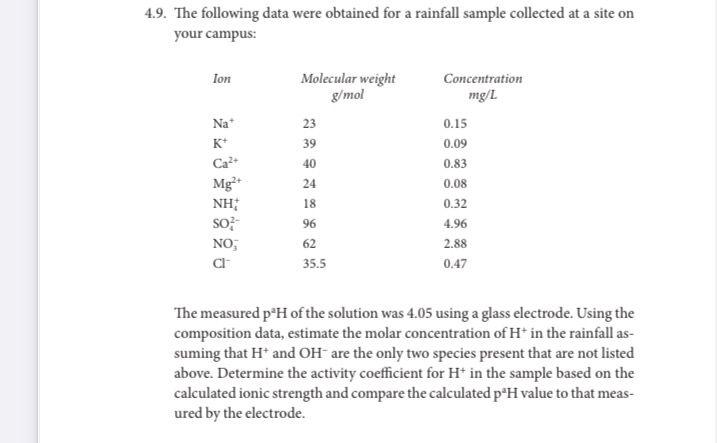
4.9. The following data were obtained for a rainfall sample collected at a site on your campus: The measured p2H of the solution was 4.05 using a glass electrode. Using the composition data, estimate the molar concentration of H+in the rainfall assuming that H+and OHare the only two species present that are not listed above. Determine the activity coefficient for H+in the sample based on the calculated ionic strength and compare the calculated p2H value to that measured by the electrode
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


