Question: ( 5 0 points ) A closed cylindrical tank ( large vessel ) of known dimensions ( L = 1 m and d = 0
points A closed cylindrical tank large vessel of known dimensions and contains Kg of colorless carbon monoxide CO gas at Evaluate the pressure exerted by the CO gas using a the ideal gas equation of state, b the generalized compressibility equation and c the Van der Waals equation. Calculate the average percent error on the pressure using the following equation
Error
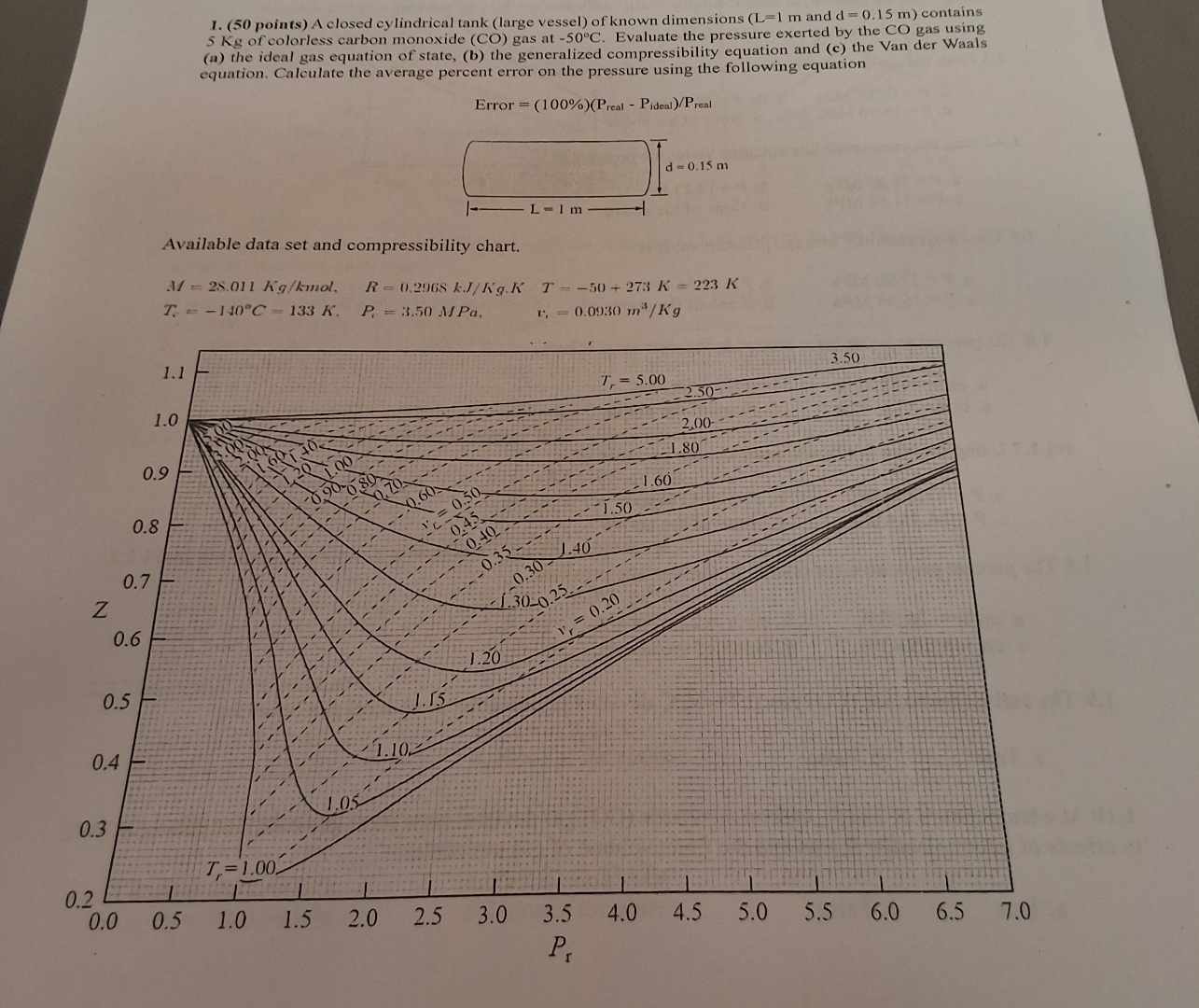
Available data set and compressibility chart.
mol,
a The ideal equation of state EOS yields the pressure as
aMPa
bkPa
cMPa
d None of the above
b From the generalized compressibility chart, verify that by marking a visible dot on it
a Done correctly
b Done incorrectly
From the compressibility chart, the corresponding pressure is
aMPa
bkPa
cMPa
d None of the above
Using the compressibility equation yields the pressure as
aMPa
bkPa
cMPa
d None of the above
The compressibility average pressure value is
aMPa
bkPa
cMPa
d None of the above
The percent pressure error related to the average pressure
a error
b error
c error
d None of the above
c Using the Van der Waals equation, the pressure is
aMPa
bkPa
cMPa
d None of the above
The percent pressure error related to the average pressure
a error
b error
c error
d None of the above
The carbon monoxide CO gas at deviates significantly from ideal gas behavior.
a True
b False
At a temperature of K carbon monoxide CO gas deviates from ideal gas behavior primarily due to effects of intermolecular forces and the finite volume of gas molecules.
a True
b False
points A closed cylindrical tank large vessel of known dimensions and contains Kg of colorless carbon monoxide CO gas at Evaluate the pressure exerted by the CO gas using a the ideal gas equation of state, b the generalized compressibility equation and c the Van der Waals equation. Calculate the average percent error on the pressure using the following equation
Error
Available data set and compressibility chart.
mol,
MPa,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


