Question: 5. (10 pts) Hemoglobin. This problem using a simplified model for the binding of oxygen (O2) to the hemoglobin molecules in your red blood cells:
5. (10 pts) Hemoglobin. This problem using a simplified model for the binding of oxygen (O2) to the hemoglobin molecules in your red blood cells: We will assume each hemoglobi
2
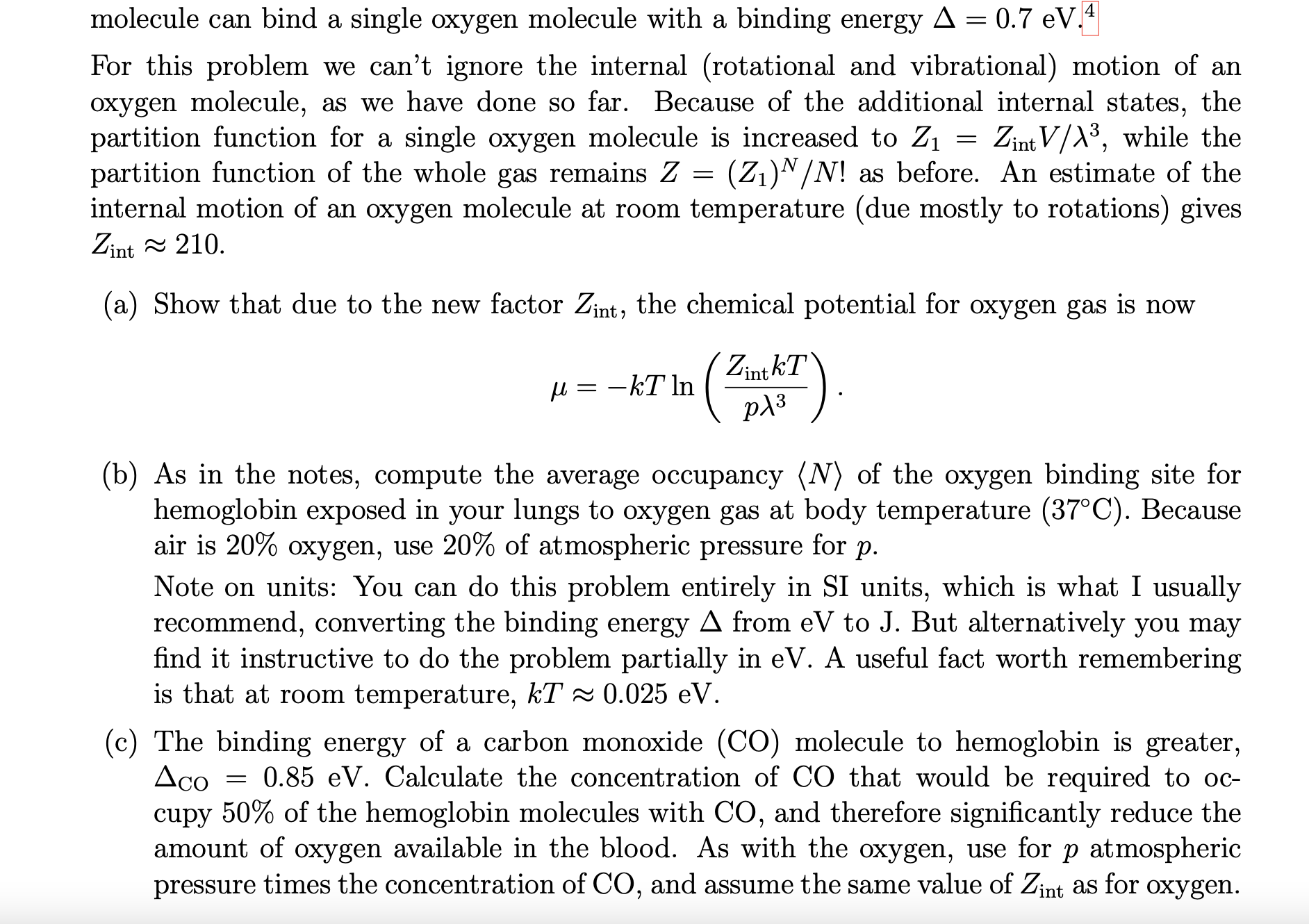
molecule can bind a single oxygen molecule with a binding energy = 0.7 eV.4
For this problem we cant ignore the internal (rotational and vibrational) motion of an oxygen molecule, as we have done so far. Because of the additional internal states, the partition function for a single oxygen molecule is increased to Z1 = Zint V /3 , while the partition function of the whole gas remains Z = (Z1)N/N! as before. An estimate of the internal motion of an oxygen molecule at room temperature (due mostly to rotations) gives Zint 210.
(a) Show that due to the new factor Zint, the chemical potential for oxygen gas is now ZintkT
(b) As in the notes, compute the average occupancy N of the oxygen binding site for hemoglobin exposed in your lungs to oxygen gas at body temperature (37C). Because air is 20% oxygen, use 20% of atmospheric pressure for p.
Note on units: You can do this problem entirely in SI units, which is what I usually recommend, converting the binding energy from eV to J. But alternatively you may find it instructive to do the problem partially in eV. A useful fact worth remembering is that at room temperature, kT 0.025 eV.
(c) The binding energy of a carbon monoxide (CO) molecule to hemoglobin is greater, CO = 0.85 eV. Calculate the concentration of CO that would be required to oc- cupy 50% of the hemoglobin molecules with CO, and therefore significantly reduce the amount of oxygen available in the blood. As with the oxygen, use for p atmospheric pressure times the concentration of CO, and assume the same value of Zint as for oxygen.

5. (10 pts) Hemoglobin. This problem using a simplified model for the binding of oxygen (O2) to the hemoglobin molecules in your red blood cells: We will assume each hemoglobin molecule can bind a single oxygen molecule with a binding energy =0.7eV.4 For this problem we can't ignore the internal (rotational and vibrational) motion of an oxygen molecule, as we have done so far. Because of the additional internal states, the partition function for a single oxygen molecule is increased to Z1=ZintV/3, while the partition function of the whole gas remains Z=(Z1)N/N ! as before. An estimate of the internal motion of an oxygen molecule at room temperature (due mostly to rotations) gives Zint210. (a) Show that due to the new factor Zint, the chemical potential for oxygen gas is now =kTln(p3ZintkT). (b) As in the notes, compute the average occupancy N of the oxygen binding site for hemoglobin exposed in your lungs to oxygen gas at body temperature (37C). Because air is 20% oxygen, use 20% of atmospheric pressure for p. Note on units: You can do this problem entirely in SI units, which is what I usually recommend, converting the binding energy from eV to J. But alternatively you may find it instructive to do the problem partially in eV. A useful fact worth remembering is that at room temperature, kT0.025eV. (c) The binding energy of a carbon monoxide (CO) molecule to hemoglobin is greater, CO=0.85eV. Calculate the concentration of CO that would be required to occupy 50% of the hemoglobin molecules with CO, and therefore significantly reduce the amount of oxygen available in the blood. As with the oxygen, use for p atmospheric pressure times the concentration of CO, and assume the same value of Zint as for oxygen. 5. (10 pts) Hemoglobin. This problem using a simplified model for the binding of oxygen (O2) to the hemoglobin molecules in your red blood cells: We will assume each hemoglobin molecule can bind a single oxygen molecule with a binding energy =0.7eV.4 For this problem we can't ignore the internal (rotational and vibrational) motion of an oxygen molecule, as we have done so far. Because of the additional internal states, the partition function for a single oxygen molecule is increased to Z1=ZintV/3, while the partition function of the whole gas remains Z=(Z1)N/N ! as before. An estimate of the internal motion of an oxygen molecule at room temperature (due mostly to rotations) gives Zint210. (a) Show that due to the new factor Zint, the chemical potential for oxygen gas is now =kTln(p3ZintkT). (b) As in the notes, compute the average occupancy N of the oxygen binding site for hemoglobin exposed in your lungs to oxygen gas at body temperature (37C). Because air is 20% oxygen, use 20% of atmospheric pressure for p. Note on units: You can do this problem entirely in SI units, which is what I usually recommend, converting the binding energy from eV to J. But alternatively you may find it instructive to do the problem partially in eV. A useful fact worth remembering is that at room temperature, kT0.025eV. (c) The binding energy of a carbon monoxide (CO) molecule to hemoglobin is greater, CO=0.85eV. Calculate the concentration of CO that would be required to occupy 50% of the hemoglobin molecules with CO, and therefore significantly reduce the amount of oxygen available in the blood. As with the oxygen, use for p atmospheric pressure times the concentration of CO, and assume the same value of Zint as for oxygen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


