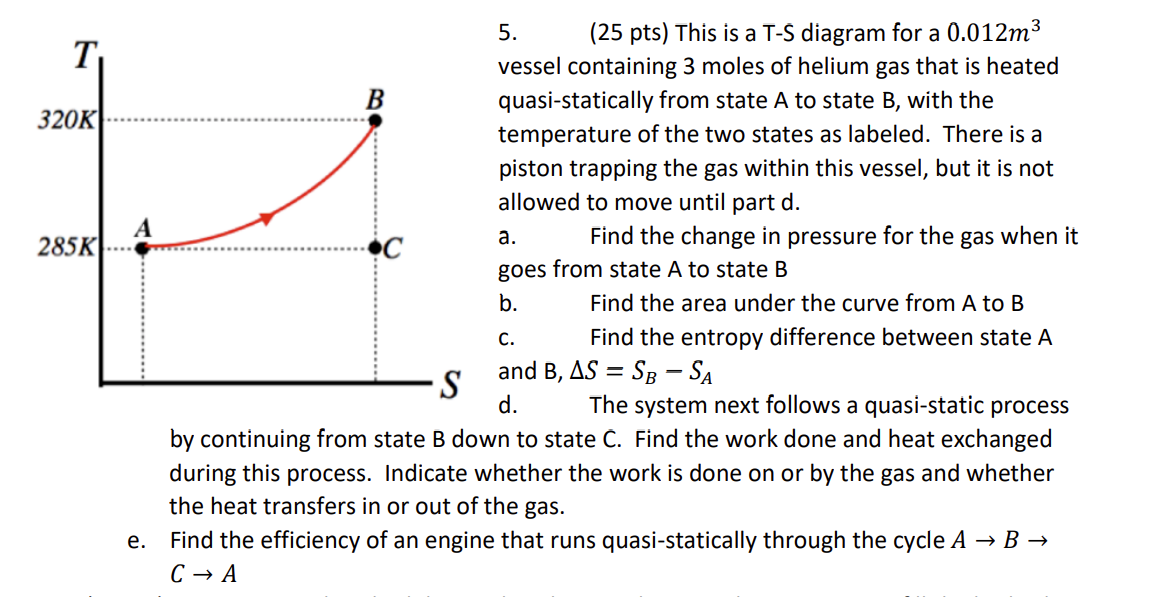
Question: 5 . ( 2 5 pts ) This is a T - S diagram for a ( 0 . 0 1 2 mathrm
pts This is a TS diagram for a mathrm~m vessel containing moles of helium gas that is heated quasistatically from state A to state B with the temperature of the two states as labeled. There is a piston trapping the gas within this vessel, but it is not allowed to move until part d
a Find the change in pressure for the gas when it goes from state A to state B
b Find the area under the curve from A to B
c Find the entropy difference between state A
d The system next follows a quasistatic process by continuing from state B down to state C Find the work done and heat exchanged during this process. Indicate whether the work is done on or by the gas and whether the heat transfers in or out of the gas.
e Find the efficiency of an engine that runs quasistatically through the cycle A rightarrow B rightarrow C rightarrow A
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


