Question: * * 5 . 2 . 9 The overall yield of a product on a substrate in lute value of the production rate divided by
The overall yield of a product on a substrate in lute value of the production rate divided by the rate of consumption of the feed in the substrate the liquid containing the cells, nutrients, etc. The overall chemical reaction for the oxidation of ethylene to epoxide is
Calculate the theoretical yield conversion of in moles per mole for Reaction a
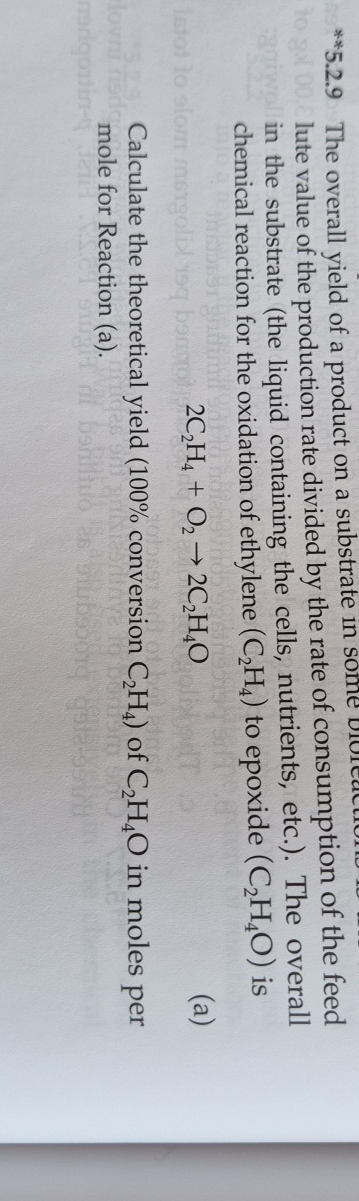
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


