Question: 5. Answer ALL parts. (a) in complex ion 1, four of the Cu-N bond lengths are 1.9 whereas two are 2.4 . In contrast, complex
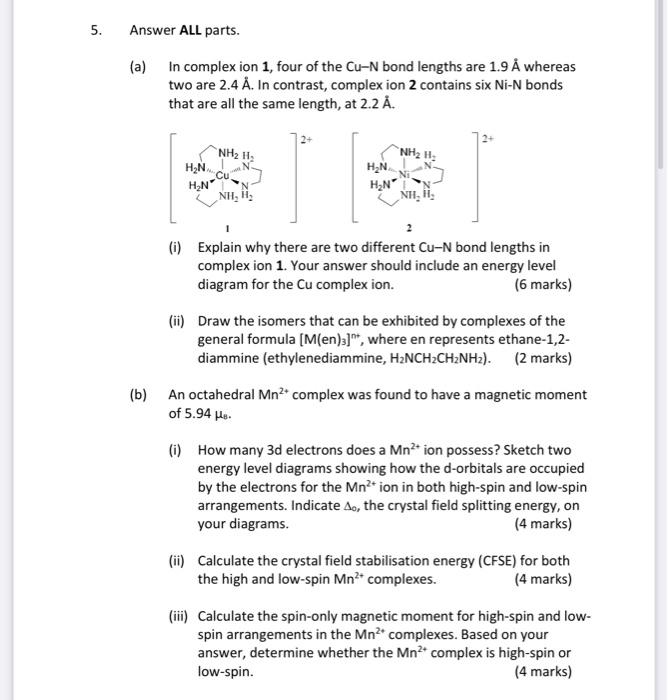
5. Answer ALL parts. (a) in complex ion 1, four of the Cu-N bond lengths are 1.9 whereas two are 2.4 . In contrast, complex ion 2 contains six Ni-N bonds that are all the same length, at 2.2 . NH2 H HN NH2H N Ni H N cu NH, li, HN HN' NH, Hi- (i) Explain why there are two different Cu-N bond lengths in complex ion 1. Your answer should include an energy level diagram for the Cu complex ion. (6 marks) (ii) Draw the isomers that can be exhibited by complexes of the general formula (M(en)3]", where en represents ethane-1,2- diammine (ethylenediammine, H2NCH2CH2NH2). (2 marks) (b) An octahedral Mn2 complex was found to have a magnetic moment of 5.94 Ho. (i) How many 3d electrons does a Mn* ion possess? Sketch two energy level diagrams showing how the d-orbitals are occupied by the electrons for the Mn?' ion in both high-spin and low-spin arrangements. Indicate A, the crystal field splitting energy, on your diagrams. (4 marks) (ii) Calculate the crystal field stabilisation energy (CFSE) for both the high and low-spin Mn * complexes. (4 marks) (iii) Calculate the spin-only magnetic moment for high-spin and low- spin arrangements in the Mn+ complexes. Based on your answer, determine whether the Mn2+ complex is high-spin or low-spin. (4 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


