Question: 5. d4RN=TH solve for T Show your work for problems 6-7. 6. If the specific heat of water is (1.00cal/g.c) how many calories of heat
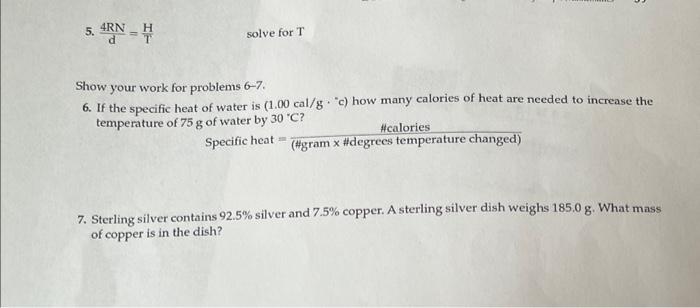
5. d4RN=TH solve for T Show your work for problems 6-7. 6. If the specific heat of water is (1.00cal/g."c) how many calories of heat are necded to increase the temperature of 75g of water by 30C ? Specific heat =(#gramx#degreestemperaturechanged)#calories 7. Sterling silver contains 92.5% silver and 7.5% copper. A sterling silver dish weighs 185.0g. What mass of copper is in the dish
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


