Question: 5) For each substance below, write its hydrolysis equation and calculate what is asked from the information provided a) the Ka of C6H5COOH (benzoic acid)
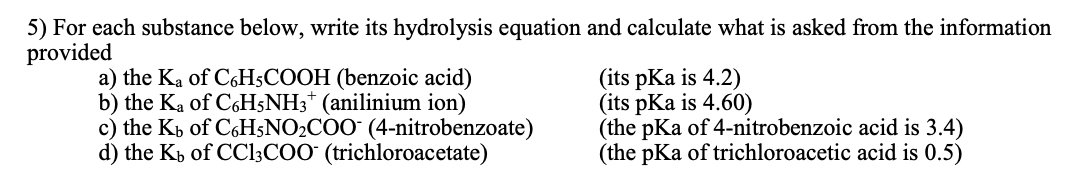
5) For each substance below, write its hydrolysis equation and calculate what is asked from the information provided a) the Ka of C6H5COOH (benzoic acid) (its pKa is 4.2) b) the Ka of C6H5NH3* (anilinium ion) (its pKa is 4.60) c) the Ky of C6H5NO2C00-(4-nitrobenzoate) (the pKa of 4-nitrobenzoic acid is 3.4) d) the Kb of CC13C0O* (trichloroacetate) (the pKa of trichloroacetic acid is 0.5)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


