Question: (5 points). Using the thermochemical data in the table below, determine the standard enthalpy of reaction (H) for the combustion of one mole of propane
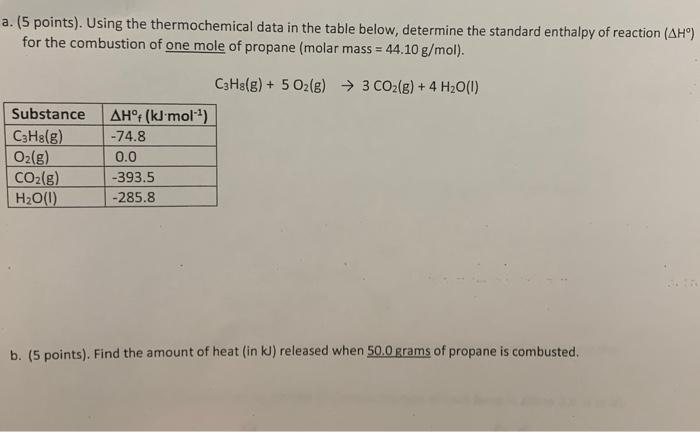
(5 points). Using the thermochemical data in the table below, determine the standard enthalpy of reaction (H) for the combustion of one mole of propane (molar mass =44.10g/mol ). C3H8(g)+5O2(g)3CO2(g)+4H2O(I) b. (5 points). Find the amount of heat (in kJ ) released when 50.0 grams of propane is combusted
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


