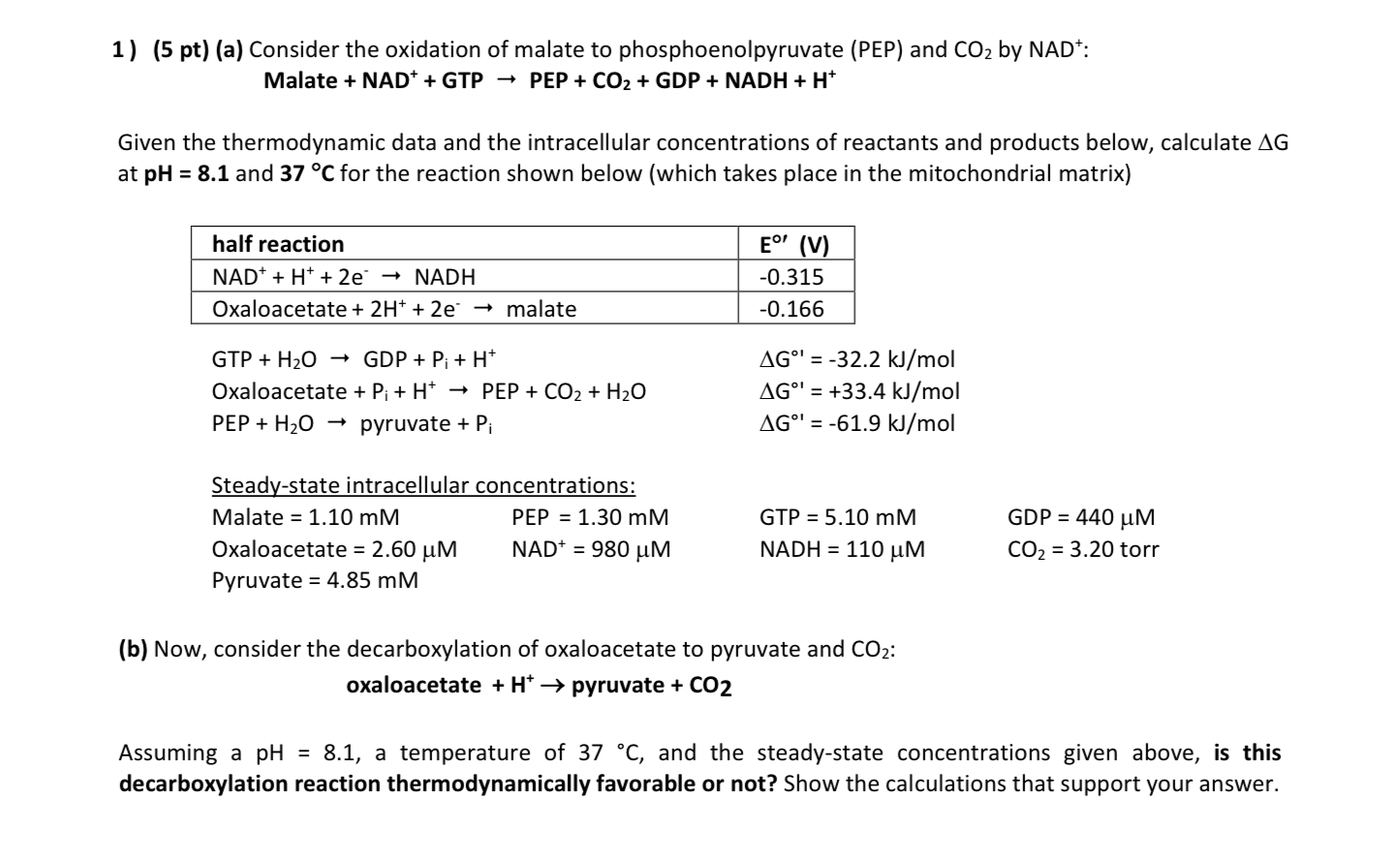
Question: ( 5 pt ) ( a ) Consider the oxidation of malate to phosphoenolpyruvate ( PEP ) and C O 2 by N A D
pta Consider the oxidation of malate to phosphoenolpyruvate PEP and by :
Malate PEPNADH
Given the thermodynamic data and the intracellular concentrations of reactants and products below, calculate
at and for the reaction shown below which takes place in the mitochondrial matrix
Oxaloacetate PEP
PEP pyruvate
Steadystate intracellular concentrations:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


