Question: 5 second UV line - is it enough time for complete polymerization of Poly(ethylene glycol) diacrylate? Data below. Poly(ethylene glycol) diacrylate: Reaction of six molecules
5 second UV line - is it enough time for complete polymerization of Poly(ethylene glycol) diacrylate? Data below.
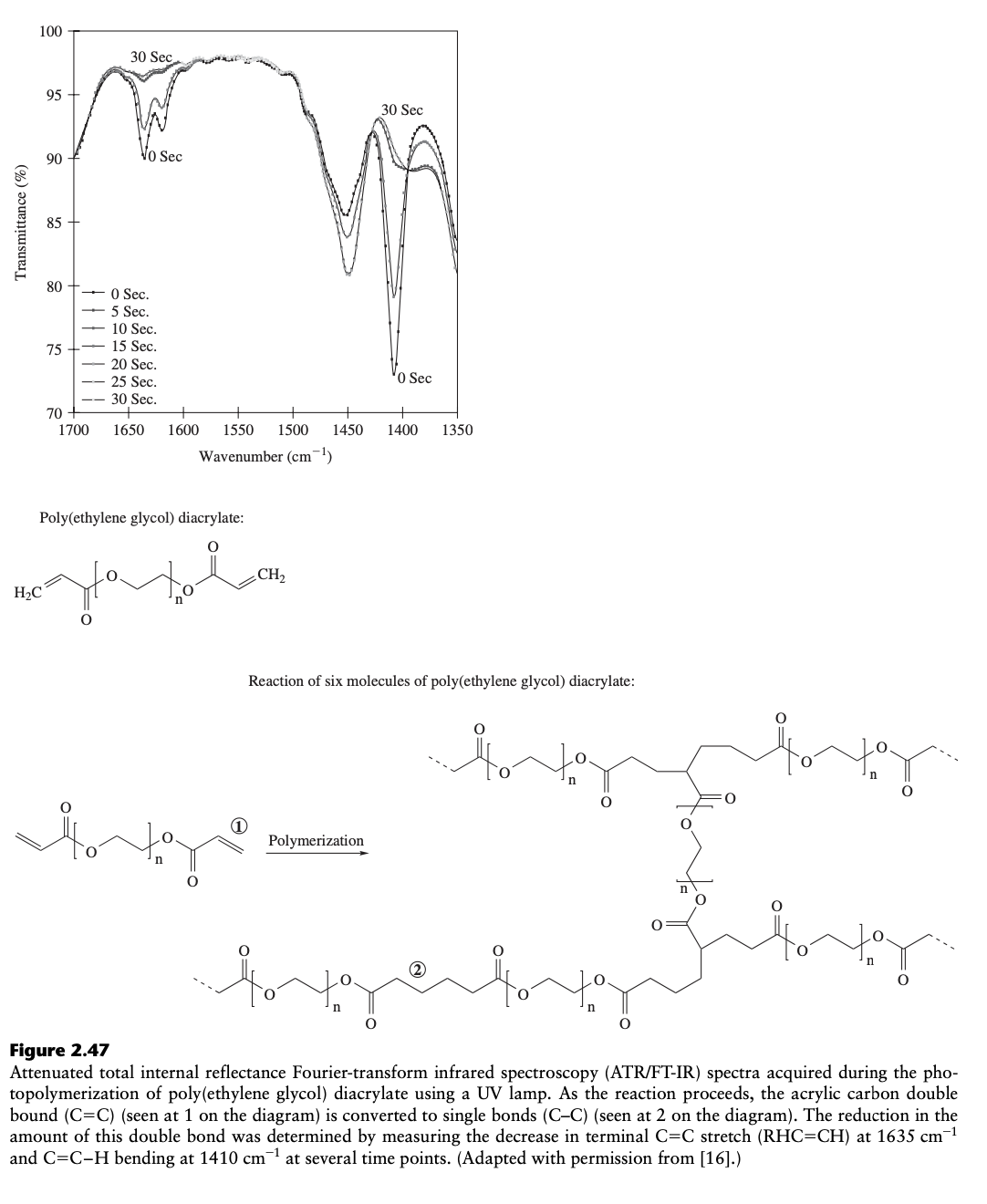
Poly(ethylene glycol) diacrylate: Reaction of six molecules of poly(ethylene glycol) diacrylate: Polymerization (2) Figure 2.47 Attenuated total internal reflectance Fourier-transform infrared spectroscopy (ATR/FT-IR) spectra acquired during the photopolymerization of poly(ethylene glycol) diacrylate using a UV lamp. As the reaction proceeds, the acrylic carbon double bound (C=C ) (seen at 1 on the diagram) is converted to single bonds (CC) (seen at 2 on the diagram). The reduction in the amount of this double bond was determined by measuring the decrease in terminal C=C stretch (RHC=CH) at 1635cm1 and C=CH bending at 1410cm1 at several time points. (Adapted with permission from [16].)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


