Question: (5%) The conversion from an adiabatic PBR is shown below as a function of temperature. Which of the following statements are TRUE? [2.5% for each
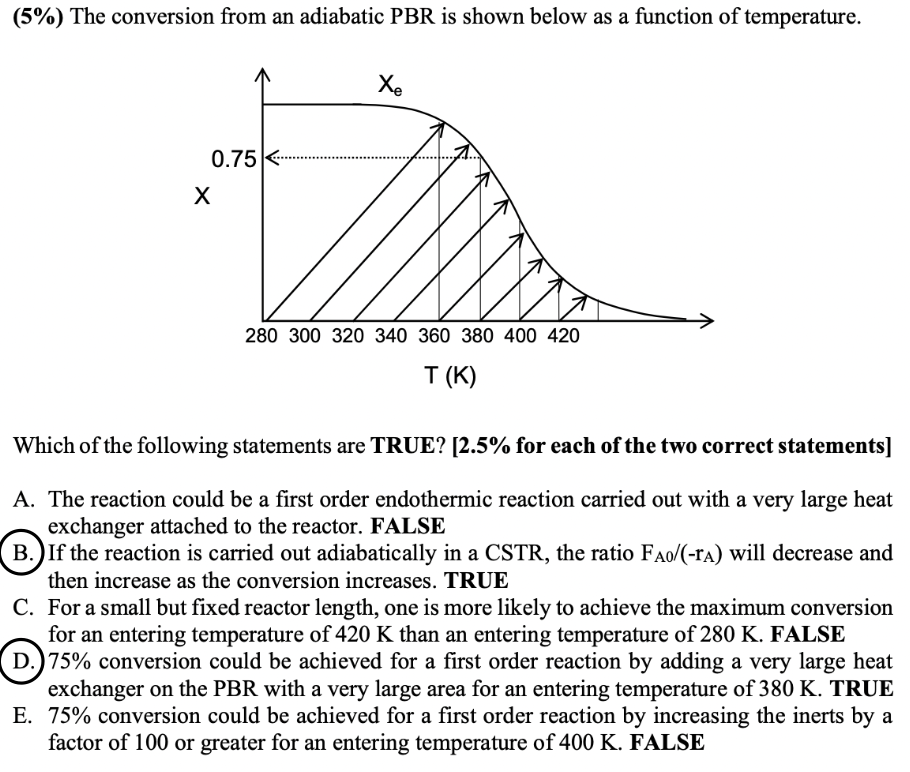
(5%) The conversion from an adiabatic PBR is shown below as a function of temperature. Which of the following statements are TRUE? [2.5\% for each of the two correct statements] A. The reaction could be a first order endothermic reaction carried out with a very large heat exchanger attached to the reactor. FALSE B. If the reaction is carried out adiabatically in a CSTR, the ratio FA0/(rA) will decrease and then increase as the conversion increases. TRUE C. For a small but fixed reactor length, one is more likely to achieve the maximum conversion for an entering temperature of 420K than an entering temperature of 280K. FALSE D. 75% conversion could be achieved for a first order reaction by adding a very large heat exchanger on the PBR with a very large area for an entering temperature of 380K. TRUE E. 75% conversion could be achieved for a first order reaction by increasing the inerts by a factor of 100 or greater for an entering temperature of 400K. FALSE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


