Question: 5) Two electrochemical cells are connected as shown. Cell A Zn(s)| Zn2+(0.85 M) || Cu2+(1.10 M)| Cu(s) Zn(s) Zn+(1.05 M)|| Cu2+(0.75 M)| Cu(s) Cell B
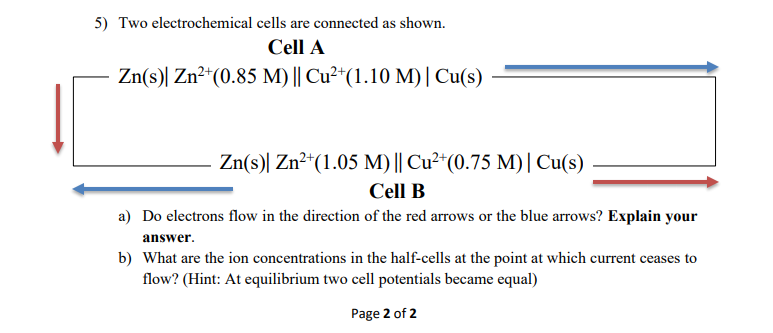
5) Two electrochemical cells are connected as shown. Cell A Zn(s)| Zn2+(0.85 M) || Cu2+(1.10 M)| Cu(s) Zn(s) Zn+(1.05 M)|| Cu2+(0.75 M)| Cu(s) Cell B a) Do electrons flow in the direction of the red arrows or the blue arrows? Explain your answer. b) What are the ion concentrations in the half-cells at the point at which current ceases to flow? (Hint: At equilibrium two cell potentials became equal) Page 2 of 2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


