Question: 5. Using the GLC trace for the alkene products provided and the method of triangulation described in the pre-lab reading to estimate peak areas. (Note:

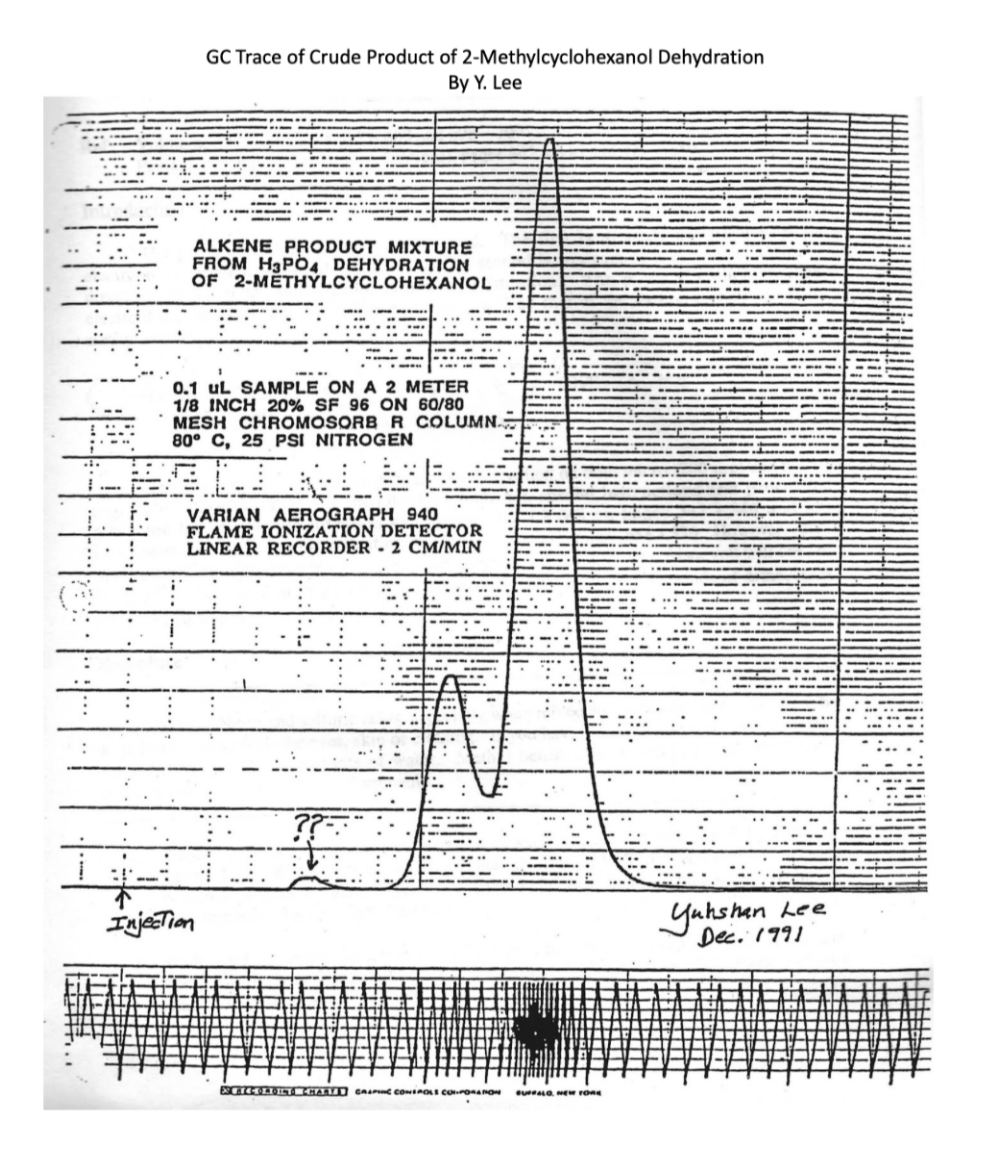
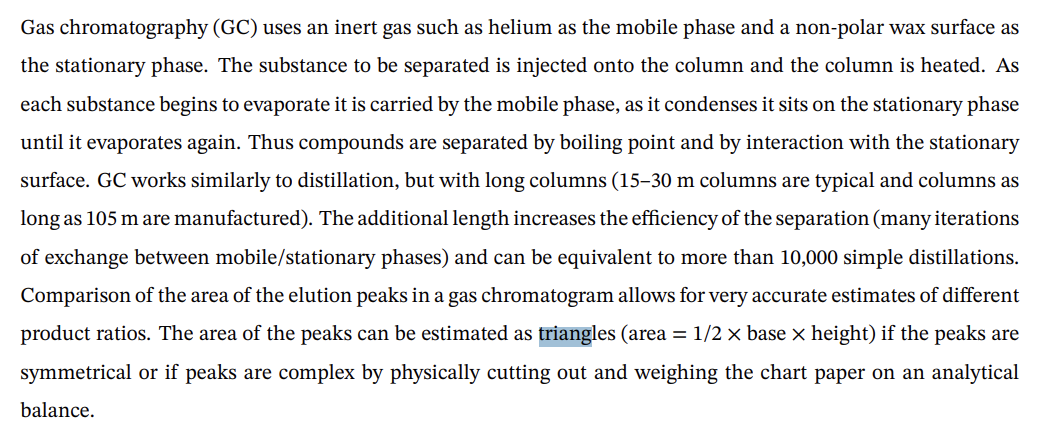
5. Using the GLC trace for the alkene products provided and the method of triangulation described in the pre-lab reading to estimate peak areas. (Note: The two peaks are overlapped. The peak marked ?? is an artifact and can be ignored.) (2pts) a. Calculate the exact ratio of 1 - and 3-methylcyclohexene products. (2pts) b. Which substance elutes more quickly? Why might that compound have a shorter retention time? GC Trace of Crude Product of 2-Methylcyclohexanol Dehydration By Y. Lee Gas chromatography (GC) uses an inert gas such as helium as the mobile phase and a non-polar wax surface as the stationary phase. The substance to be separated is injected onto the column and the column is heated. As each substance begins to evaporate it is carried by the mobile phase, as it condenses it sits on the stationary phase until it evaporates again. Thus compounds are separated by boiling point and by interaction with the stationary surface. GC works similarly to distillation, but with long columns (15-30 m columns are typical and columns as long as 105m are manufactured). The additional length increases the efficiency of the separation (many iterations of exchange between mobile/stationary phases) and can be equivalent to more than 10,000 simple distillations. Comparison of the area of the elution peaks in a gas chromatogram allows for very accurate estimates of different product ratios. The area of the peaks can be estimated as triangles (area =1/2 base height) if the peaks are symmetrical or if peaks are complex by physically cutting out and weighing the chart paper on an analytical balance
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


