Question: 5. What does a higher specific heat indicate the relationship between the amount of energy gained and the temperature change when a substance is heated?
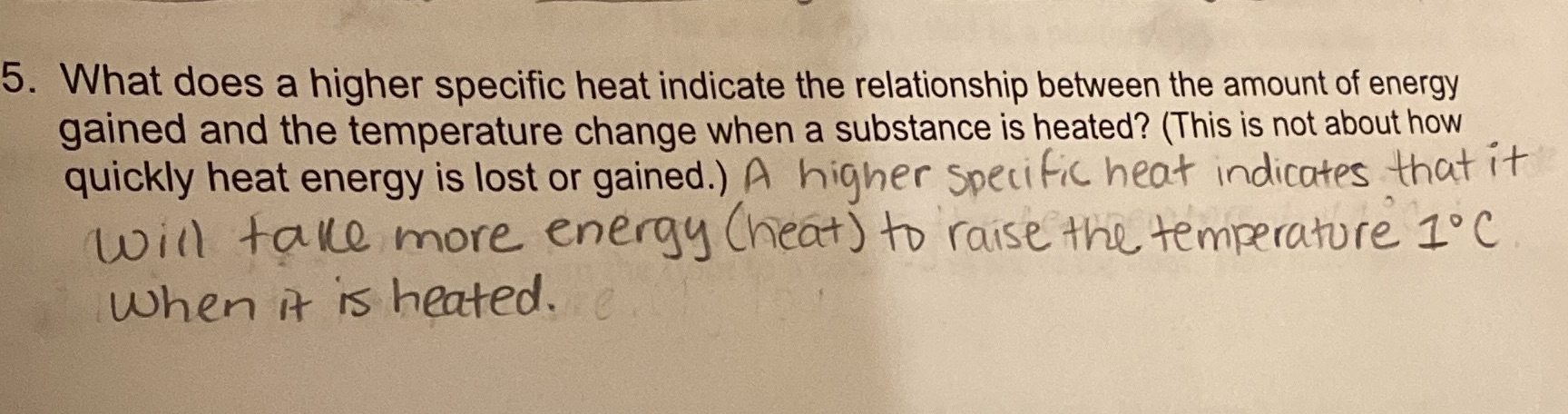
5. What does a higher specific heat indicate the relationship between the amount of energy gained and the temperature change when a substance is heated? (This is not about how quickly heat energy is lost or gained.) A higher specific heat indicates that it will take more energy (heat) to raise the temperature IC When it is heated
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


