Question: 5. Which species is paramagnetic in its standard state? (A) O2 (B) H2 (C) S8 (D) Zn (E) Cl2 6. Use molecular orbital theory to
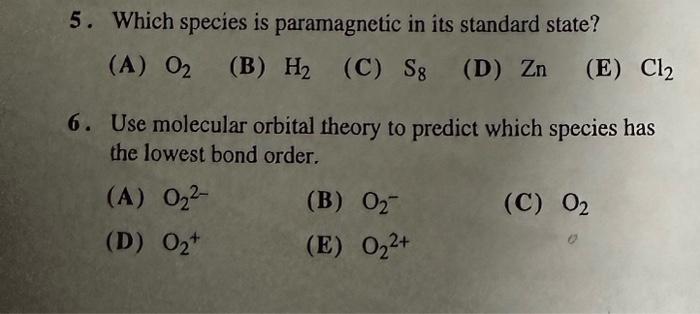
5. Which species is paramagnetic in its standard state? (A) O2 (B) H2 (C) S8 (D) Zn (E) Cl2 6. Use molecular orbital theory to predict which species has the lowest bond order. (A) O22 (B) O2 (C) O2 (D) O2+ (E) O22+
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


