Question: 5) Why is the mechanism/reaction below wrong? a) Because the bromide anion is a poor nucleophile. b) Because the hydroxide anion is a very poor
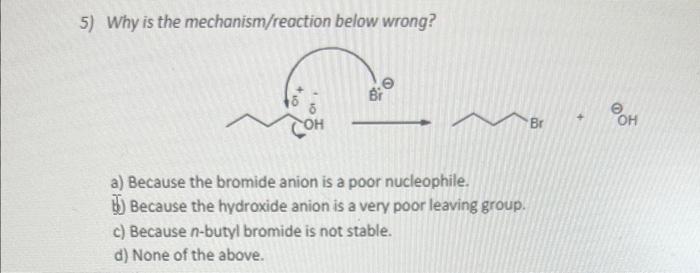
5) Why is the mechanism/reaction below wrong? a) Because the bromide anion is a poor nucleophile. b) Because the hydroxide anion is a very poor leaving group. c) Because n-butyl bromide is not stable. d) None of the above
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


