Question: 5.49. Using Henry's law, determine the difference between the freezing point of pure water and water saturated with air at 1 atm. For N2 at
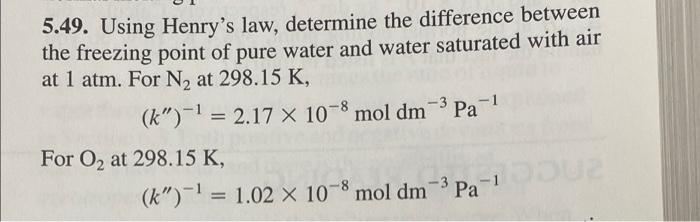
5.49. Using Henry's law, determine the difference between the freezing point of pure water and water saturated with air at 1 atm. For N2 at 298.15 K, (k")-1 = 2.17 x 10-8 mol dm-3 Pa-1 = For 02 at 298.15 K, (k)-1 = 1.02 X 10-8 mol dm-Pa-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


