Question: 586 m/s 713 m/s Question 5 (1 point) When 100. mL of 1.00 M Ba(NO3)2 solution is mixed with 100 mL of 1.00 M Na2SO4
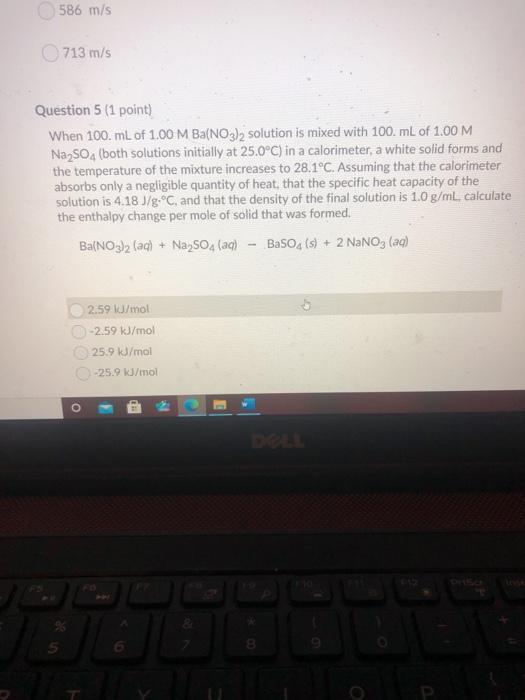
586 m/s 713 m/s Question 5 (1 point) When 100. mL of 1.00 M Ba(NO3)2 solution is mixed with 100 mL of 1.00 M Na2SO4 (both solutions initially at 25.0C) in a calorimeter, a white solid forms and the temperature of the mixture increases to 28.1C. Assuming that the calorimeter absorbs only a negligible quantity of heat, that the specific heat capacity of the solution is 4.18 J/g.C, and that the density of the final solution is 1.0 g/mL. calculate the enthalpy change per mole of solid that was formed. Ba(NO3)2 (aq) + Na2SO4 (aq) BaSO4(s) + 2 NaNO3 (ac) 2.59 kJ/mol -2.59 kJ/mol 25.9 kJ/mol -25.9 kJ/mol o E 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


