Question: 6. {15} The data in the table below were obtained using the initial rate method for the reaction: 2NO(g)+2O2(g)2NO2(g)) a) {6} analyze data and deduce
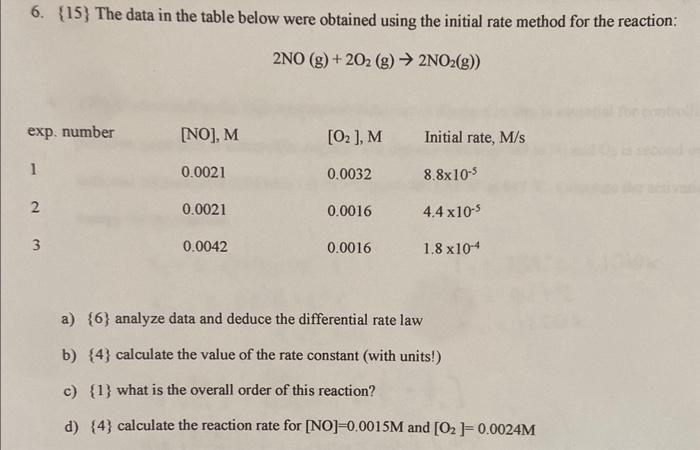
6. {15} The data in the table below were obtained using the initial rate method for the reaction: 2NO(g)+2O2(g)2NO2(g)) a) {6} analyze data and deduce the differential rate law b) {4} calculate the value of the rate constant (with units!) c) {1} what is the overall order of this reaction? d) {4} calculate the reaction rate for [NO]=0.0015M and [O2]=0.0024M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


