Question: 6 4. (a) Given the following experimental data: H2(g) + F2 (g) 2HF (g) AH, = -537 kJ C(s) + 2F2 (g) CF4 (8) AH,
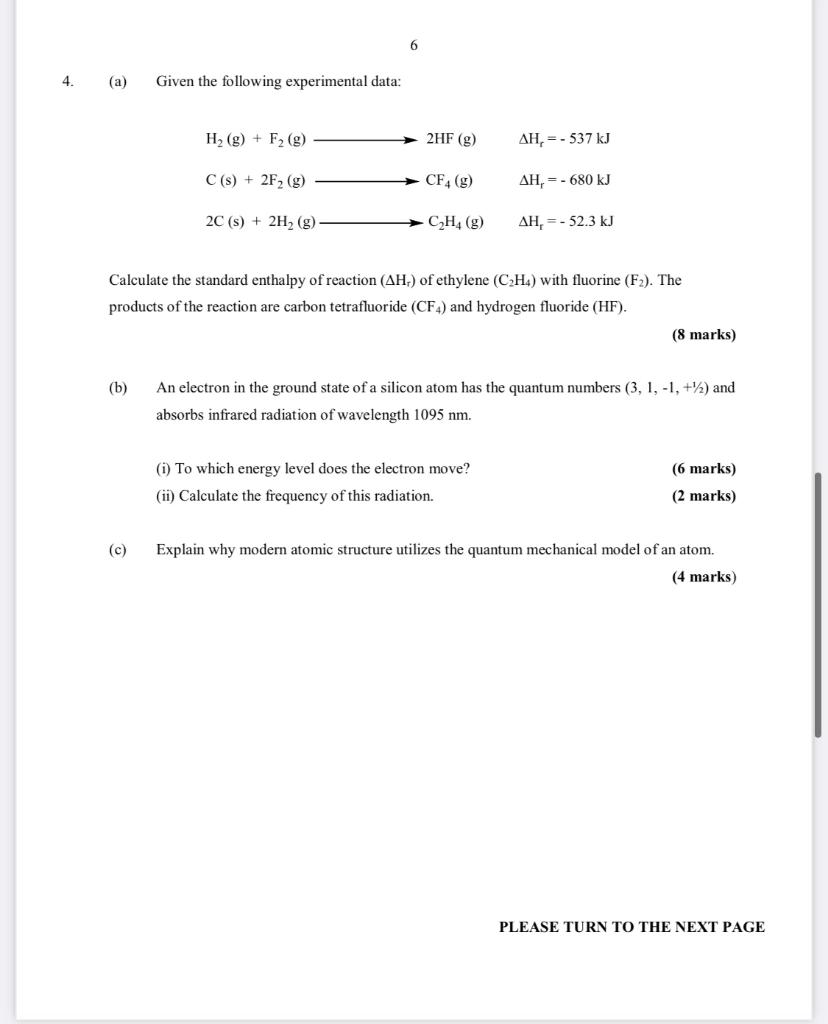
6 4. (a) Given the following experimental data: H2(g) + F2 (g) 2HF (g) AH, = -537 kJ C(s) + 2F2 (g) CF4 (8) AH, = - 680 kJ 2C(s) + 2H2 (g) C2H4 (g) AH, = -52.3 kJ Calculate the standard enthalpy of reaction (AHL) of ethylene (CH4) with fluorine (F2). The products of the reaction are carbon tetrafluoride (CF4) and hydrogen fluoride (HF). (8 marks) (b) An electron in the ground state of a silicon atom has the quantum numbers (3, 1,-1, +) and absorbs infrared radiation of wavelength 1095 nm. (i) To which energy level does the electron move? (ii) Calculate the frequency of this radiation. (6 marks) (2 marks) (c) Explain why modern atomic structure utilizes the quantum mechanical model of an atom. (4 marks) PLEASE TURN TO THE NEXT PAGE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


