Question: 6, 7 and 8 PLEASE! 6. The equation for the line on the plot below is y=9.29x103+23.85. Which one of the following quantities (and its
6, 7 and 8 PLEASE!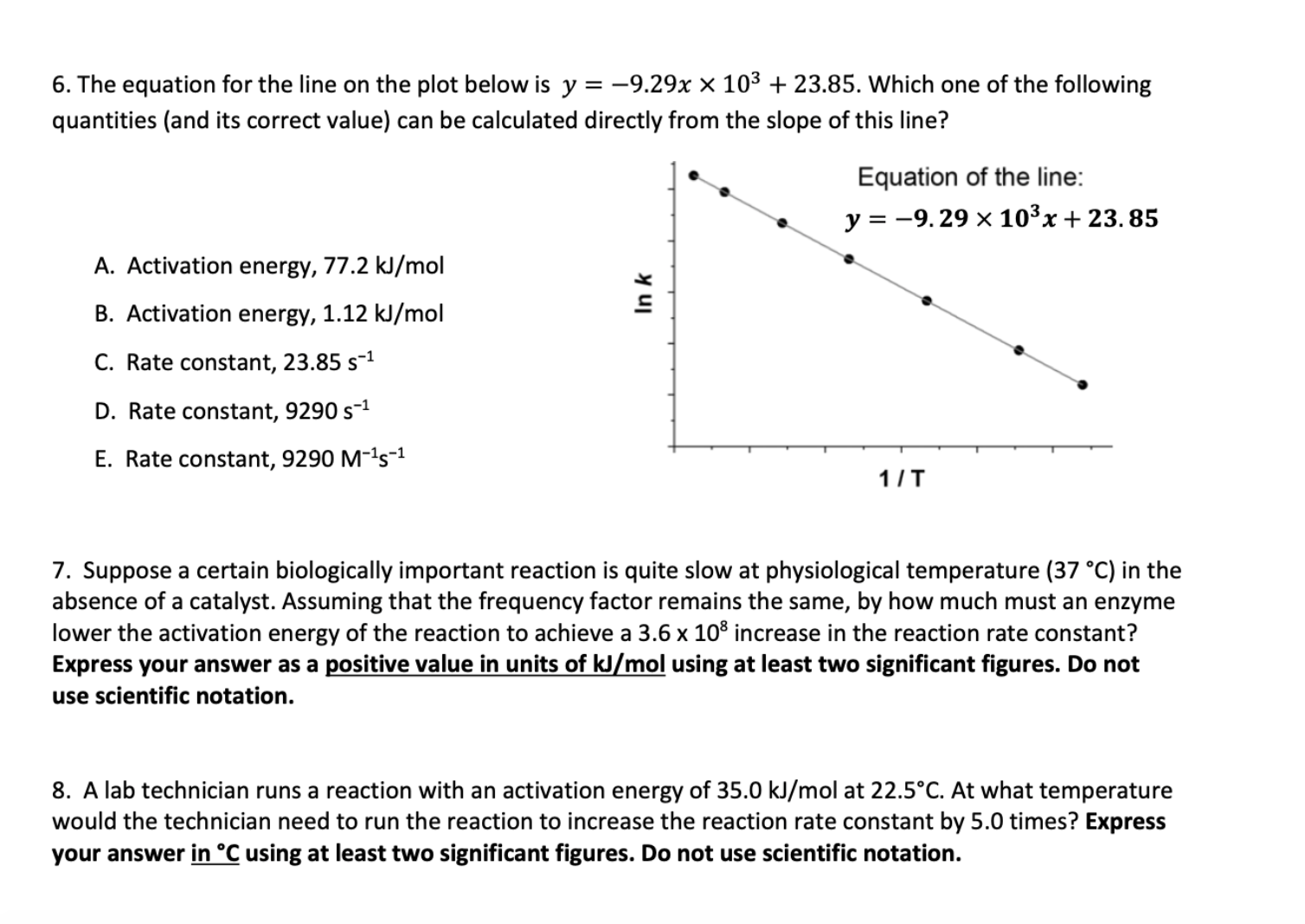
6. The equation for the line on the plot below is y=9.29x103+23.85. Which one of the following quantities (and its correct value) can be calculated directly from the slope of this line? A. Activation energy, 77.2kJ/mol B. Activation energy, 1.12kJ/mol C. Rate constant, 23.85s1 D. Rate constant, 9290s1 E. Rate constant, 9290M1s1 7. Suppose a certain biologically important reaction is quite slow at physiological temperature (37C) in the absence of a catalyst. Assuming that the frequency factor remains the same, by how much must an enzyme lower the activation energy of the reaction to achieve a 3.6108 increase in the reaction rate constant? Express your answer as a positive value in units of kJ/mol using at least two significant figures. Do not use scientific notation. 8. A lab technician runs a reaction with an activation energy of 35.0kJ/mol at 22.5C. At what temperature would the technician need to run the reaction to increase the reaction rate constant by 5.0 times? Express your answer in C using at least two significant figures. Do not use scientific notation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


