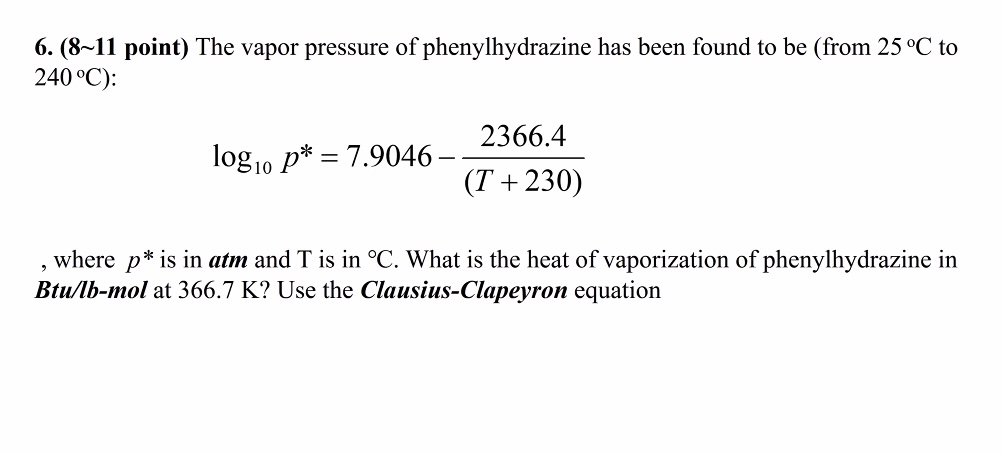
Question: 6 . ( 8 1 1 point ) The vapor pressure of phenylhydrazine has been found to be ( from 2 5 C to (
point The vapor pressure of phenylhydrazine has been found to be from to :
where is in atm and is in What is the heat of vaporization of phenylhydrazine in Btulbmol at K Use the ClausiusClapeyron equation
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


