Question: 6) A solid chloride sample weighs 0.3057 grams. When titrated (1 point) with a 1.207101MAgNO3 solution in a conductometric titration to precipitate AgCl,43.19mL of that
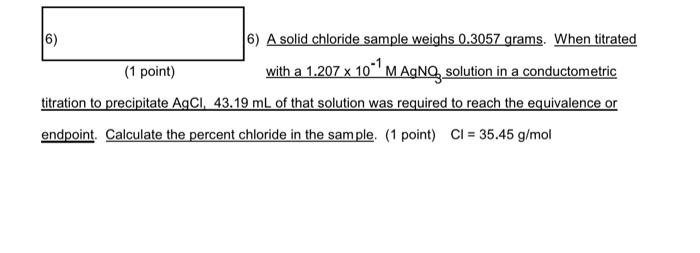
6) A solid chloride sample weighs 0.3057 grams. When titrated (1 point) with a 1.207101MAgNO3 solution in a conductometric titration to precipitate AgCl,43.19mL of that solution was required to reach the equivalence or endpoint. Calculate the percent chloride in the sample. (1 point) Cl=35.45g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


