Question: 6. Create a graph of how the potential different between the Cu and Zn electrodes will change as a function of time for Electrochemical Cell
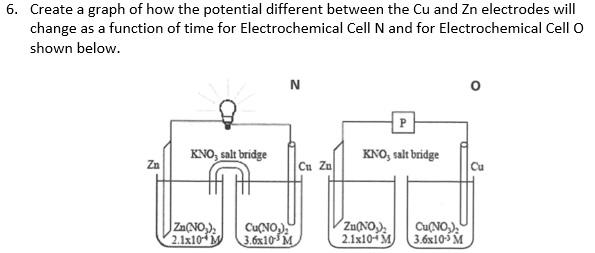
6. Create a graph of how the potential different between the Cu and Zn electrodes will change as a function of time for Electrochemical Cell N and for Electrochemical Cell O shown below. N P KNO, salt bridge Zn KNO, salt bridge Cu Zo Cu Zn(NO) 2.1x10M Cu(NO) 3.6x10 M Zu(NO) 2.1x10-4M Cu(NO) 3.6x105 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


