Question: 6. Given below are the oxides : Na2O, As2O3, N2O, NO and ChO7 Number of amphoteric oxides is: (a) 0 (b) 1 7. Match List
6. Given below are the oxides :
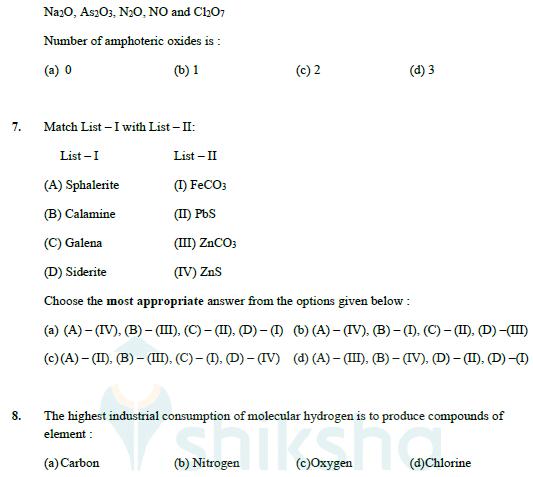

Na2O, As2O3, N2O, NO and ChO7 Number of amphoteric oxides is: (a) 0 (b) 1 7. Match List -I with List - II: List-I List-II (A) Sphalerite (I) FeCO3 (B) Calamine (II) PbS (C) Galena (III) ZnCO3 (c) 2 (d) 3 (D) Siderite (IV) ZnS Choose the most appropriate answer from the options given below: (a) (A)-(IV), (B) - (III), (C) - (II), (D)-(I) (b) (A)-(IV). (B)-(I). (C)-(II). (D) -(III) (c)(A)-(II). (B)-(III), (C) - (I), (D)-(IV) (d) (A)-(III), (B) - (IV), (D) - (II), (D) -(I) 8. The highest industrial consumption of molecular hydrogen is to produce compounds of element: (a) Carbon Nitrogen (c)Oxygen (d)Chlorine
Step by Step Solution
There are 3 Steps involved in it
The oxides listed are Na2O sodium oxide As2O3 arsenic trioxide N2O nitrous oxide NO nitric oxide Cl2... View full answer

Get step-by-step solutions from verified subject matter experts


