Question: 6. Given the following data: CO(g) + H O(g) + CO2(g) + H 2(g)2 0 -1 ( AH (25C) = -42.0 kJmol? = -1 Cp
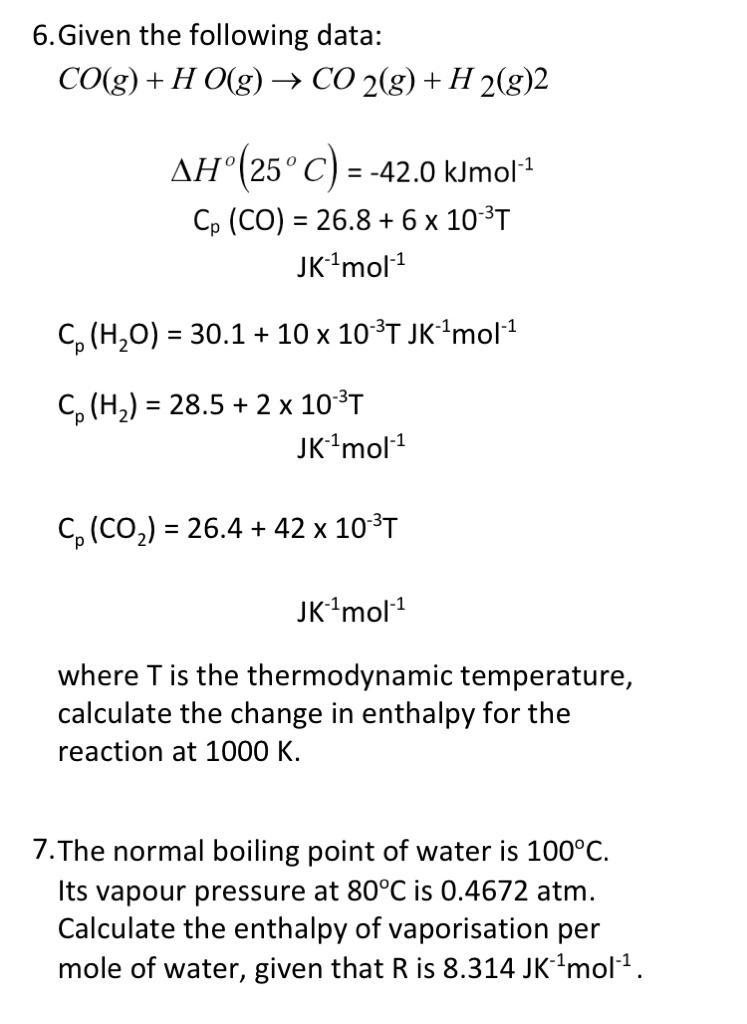
6. Given the following data: CO(g) + H O(g) + CO2(g) + H 2(g)2 0 -1 ( AH (25C) = -42.0 kJmol? = -1 Cp (CO) = 26.8 + 6 x 10-3T JK-1mol-1 Co (H2O) = 30.1 + 10 x 10-3T JK-Imol-1 = X Cp (H2) = 28.5 + 2 x 10-3T JK-1mol-1 Co (CO2) = 26.4 + 42 x 103T = JK-1mol-1 where T is the thermodynamic temperature, calculate the change in enthalpy for the reaction at 1000 K. 7. The normal boiling point of water is 100C. Its vapour pressure at 80C is 0.4672 atm. Calculate the enthalpy of vaporisation per mole of water, given that Ris 8.314 JK-Imol-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


