Question: + 6 HNO3 H2SO4 + 6 NO2 + 2 H2O In the above equation, how many grams of water can be made when 12.8 moles
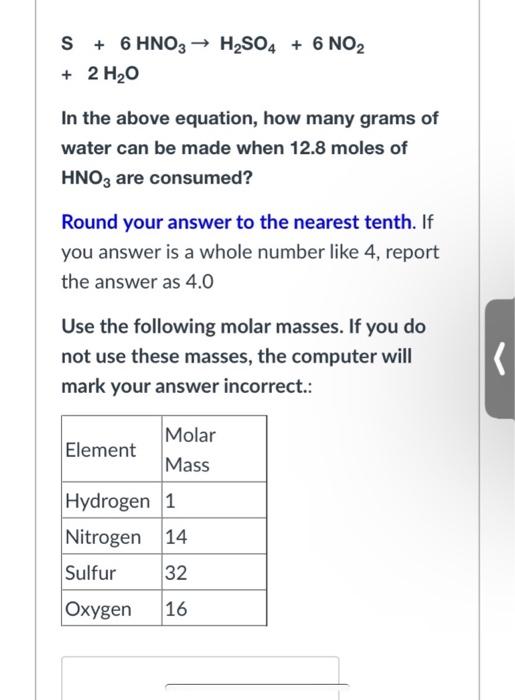
+ 6 HNO3 H2SO4 + 6 NO2 + 2 H2O In the above equation, how many grams of water can be made when 12.8 moles of HNO3 are consumed? Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0 Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.: Element Molar Mass Hydrogen 1 Nitrogen 14 Sulfur 32 Oxygen 16
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


